Abstract
Background—The frequency with which non-steroidal anti-inflammatory drugs (NSAIDs) increase small intestinal permeability and cause inflammation is uncertain. Aims—To examine small intestinal permeability and inflammation in a large number of patients on long term NSAIDs. Methods—Sixty eight patients receiving six different NSAIDs for over six months underwent combined absorption-permeability tests at three different test dose osmolarities (iso-, hypo-, and hyperosmolar). Two hundred and eighty six patients on 12 different NSAIDs underwent indium-111 white cell faecal excretion studies to assess the prevalence and severity of intestinal inflammation. Results—The iso- and hyperosmolar tests showed significant malabsorption of 3-0-methyl-D-glucose, D-xylose, and L-rhamnose. Intestinal permeability changes were significantly more pronounced and frequent with the hypo- and hyperosmolar as opposed to the iso-osmolar test. Sequential studies showed that four and nine patients (of 13) developed inflammation after three and six months treatment with NSAIDs, respectively. There was no significant difference (p>0.1) in the prevalence (54-72%) or severity of intestinal inflammation in the 286 patients taking the various NSAIDs apart from those on aspirin and nabumetone, these having no evidence of intestinal inflammation. There was no significant correlation between the inflammatory changes and age, sex, dose of NSAID, length of disease, or NSAID ingestion. Conclusions—Intestinal permeability test dose composition is an important factor when assessing the effects of NSAIDs on intestinal integrity. All the conventional NSAIDs studied were equally associated with small intestinal inflammation apart from aspirin and nabumetone which seem to spare the small bowel.
Keywords: non-steroidal anti-inflammatory drug; intestinal permeability; intestinal inflammation; aspirin; nabumetone
Full Text
The Full Text of this article is available as a PDF (127.6 KB).
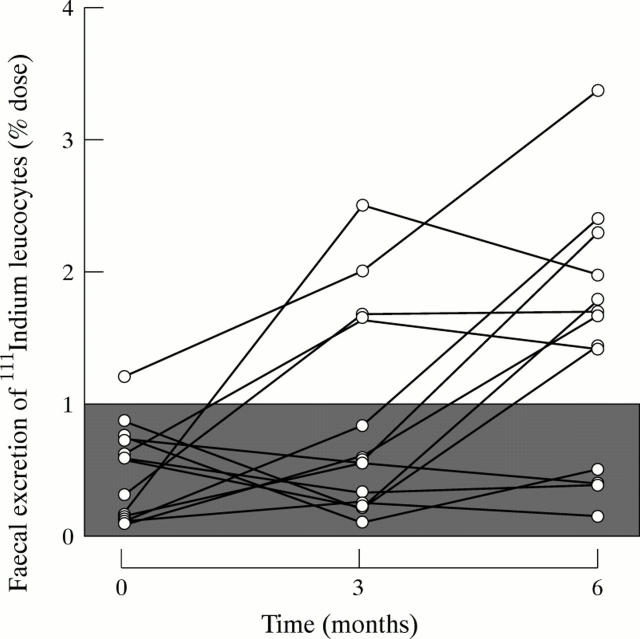
Figure 1 .
Sequential changes in the four day faecal excretion of 111In white cells in response to indomethacin or piroxicam ingestion. The shaded area represents the normal range of excretion of the labelled cells.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. P., Blower A. L. Non-steroidal anti-inflammatory drugs and life threatening complications of peptic ulceration. Gut. 1987 May;28(5):527–532. doi: 10.1136/gut.28.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardhan K. D., Bjarnason I., Scott D. L., Griffin W. M., Fenn G. C., Shield M. J., Morant S. V. The prevention and healing of acute non-steroidal anti-inflammatory drug-associated gastroduodenal mucosal damage by misoprostol. Br J Rheumatol. 1993 Nov;32(11):990–995. doi: 10.1093/rheumatology/32.11.990. [DOI] [PubMed] [Google Scholar]
- Barrier C. H., Hirschowitz B. I. Controversies in the detection and management of nonsteroidal antiinflammatory drug-induced side effects of the upper gastrointestinal tract. Arthritis Rheum. 1989 Jul;32(7):926–932. [PubMed] [Google Scholar]
- Beardon P. H., Brown S. V., McDevitt D. G. Gastrointestinal events in patients prescribed non-steroidal anti-inflammatory drugs: a controlled study using record linkage in Tayside. Q J Med. 1989 Jun;71(266):497–505. [PubMed] [Google Scholar]
- Bjarnason I., Fehilly B., Smethurst P., Menzies I. S., Levi A. J. Importance of local versus systemic effects of non-steroidal anti-inflammatory drugs in increasing small intestinal permeability in man. Gut. 1991 Mar;32(3):275–277. doi: 10.1136/gut.32.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I., Hayllar J., MacPherson A. J., Russell A. S. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology. 1993 Jun;104(6):1832–1847. doi: 10.1016/0016-5085(93)90667-2. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., MacPherson A., Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995 May;108(5):1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Maxton D., Reynolds A. P., Catt S., Peters T. J., Menzies I. S. Comparison of four markers of intestinal permeability in control subjects and patients with coeliac disease. Scand J Gastroenterol. 1994 Jul;29(7):630–639. doi: 10.3109/00365529409092484. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Smethurst P., Fenn C. G., Lee C. E., Menzies I. S., Levi A. J. Misoprostol reduces indomethacin-induced changes in human small intestinal permeability. Dig Dis Sci. 1989 Mar;34(3):407–411. doi: 10.1007/BF01536263. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Williams P., Smethurst P., Peters T. J., Levi A. J. Effect of non-steroidal anti-inflammatory drugs and prostaglandins on the permeability of the human small intestine. Gut. 1986 Nov;27(11):1292–1297. doi: 10.1136/gut.27.11.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I., Williams P., So A., Zanelli G. D., Levi A. J., Gumpel J. M., Peters T. J., Ansell B. Intestinal permeability and inflammation in rheumatoid arthritis: effects of non-steroidal anti-inflammatory drugs. Lancet. 1984 Nov 24;2(8413):1171–1174. doi: 10.1016/s0140-6736(84)92739-9. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Zanelli G., Smith T., Prouse P., Williams P., Smethurst P., Delacey G., Gumpel M. J., Levi A. J. Nonsteroidal antiinflammatory drug-induced intestinal inflammation in humans. Gastroenterology. 1987 Sep;93(3):480–489. doi: 10.1016/0016-5085(87)90909-7. [DOI] [PubMed] [Google Scholar]
- Brune K., Dietzel K., Nürnberg B., Schneider H. T. Recent insight into the mechanism of gastrointestinal tract ulceration. Scand J Rheumatol Suppl. 1987;65:135–140. doi: 10.3109/03009748709102192. [DOI] [PubMed] [Google Scholar]
- Brune K., Schweitzer A., Eckert H. Parietal cells of the stomach trap salicylates during absorption. Biochem Pharmacol. 1977 Sep 15;26(18):1735–1740. doi: 10.1016/0006-2952(77)90155-1. [DOI] [PubMed] [Google Scholar]
- Dyer N. H., Kendall M. J., Hawkins C. F. Malabsorption in rheumatoid disease. Ann Rheum Dis. 1971 Nov;30(6):626–630. doi: 10.1136/ard.30.6.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn G. C. Review article: controversies in NSAID-induced gastroduodenal damage--do they matter? Aliment Pharmacol Ther. 1994 Feb;8(1):15–26. doi: 10.1111/j.1365-2036.1994.tb00156.x. [DOI] [PubMed] [Google Scholar]
- Fries J. F., Miller S. R., Spitz P. W., Williams C. A., Hubert H. B., Bloch D. A. Toward an epidemiology of gastropathy associated with nonsteroidal antiinflammatory drug use. Gastroenterology. 1989 Feb;96(2 Pt 2 Suppl):647–655. doi: 10.1016/s0016-5085(89)80061-7. [DOI] [PubMed] [Google Scholar]
- García Rodríguez L. A., Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994 Mar 26;343(8900):769–772. doi: 10.1016/s0140-6736(94)91843-0. [DOI] [PubMed] [Google Scholar]
- Hawkey C. J. Non-steroidal anti-inflammatory drugs and peptic ulcers. BMJ. 1990 Feb 3;300(6720):278–284. doi: 10.1136/bmj.300.6720.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayllar J., Macpherson A., Bjarnason I. Gastroprotection and nonsteroidal anti-inflammatory drugs (NSAIDS). Rationale and clinical implications. Drug Saf. 1992 Mar-Apr;7(2):86–105. doi: 10.2165/00002018-199207020-00002. [DOI] [PubMed] [Google Scholar]
- Hayllar J., Smith T., Macpherson A., Price A. B., Gumpel M., Bjarnason I. Nonsteroidal antiinflammatory drug-induced small intestinal inflammation and blood loss. Effects of sulfasalazine and other disease-modifying antirheumatic drugs. Arthritis Rheum. 1994 Aug;37(8):1146–1150. doi: 10.1002/art.1780370806. [DOI] [PubMed] [Google Scholar]
- Henry D., Lim L. L., Garcia Rodriguez L. A., Perez Gutthann S., Carson J. L., Griffin M., Savage R., Logan R., Moride Y., Hawkey C. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. BMJ. 1996 Jun 22;312(7046):1563–1566. doi: 10.1136/bmj.312.7046.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins A. P., Trew D. R., Crump B. J., Nukajam W. S., Foley J. A., Menzies I. S., Creamer B. Do non-steroidal anti-inflammatory drugs increase colonic permeability? Gut. 1991 Jan;32(1):66–69. doi: 10.1136/gut.32.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juby L. D., Axon A. T., Wright V., Winstanley P., Rothwell J. Intestinal permeability and inflammation in rheumatoid arthritis. Br J Rheumatol. 1986 May;25(2):226–227. doi: 10.1093/rheumatology/25.2.226-a. [DOI] [PubMed] [Google Scholar]
- Keating J., Bjarnason I., Somasundaram S., Macpherson A., Francis N., Price A. B., Sharpstone D., Smithson J., Menzies I. S., Gazzard B. G. Intestinal absorptive capacity, intestinal permeability and jejunal histology in HIV and their relation to diarrhoea. Gut. 1995 Nov;37(5):623–629. doi: 10.1136/gut.37.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laker M. F., Menzies I. S. Increase in human intestinal permeability following ingestion of hypertonic solutions. J Physiol. 1977 Mar;265(3):881–894. doi: 10.1113/jphysiol.1977.sp011750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenbach R., Morham S. G., Tiano H. F., Loftin C. D., Ghanayem B. I., Chulada P. C., Mahler J. F., Lee C. A., Goulding E. H., Kluckman K. D. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995 Nov 3;83(3):483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- Langman M. J., Weil J., Wainwright P., Lawson D. H., Rawlins M. D., Logan R. F., Murphy M., Vessey M. P., Colin-Jones D. G. Risks of bleeding peptic ulcer associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994 Apr 30;343(8905):1075–1078. doi: 10.1016/s0140-6736(94)90185-6. [DOI] [PubMed] [Google Scholar]
- Lanza F. L. A review of gastric ulcer and gastroduodenal injury in normal volunteers receiving aspirin and other non-steroidal anti-inflammatory drugs. Scand J Gastroenterol Suppl. 1989;163:24–31. doi: 10.3109/00365528909091171. [DOI] [PubMed] [Google Scholar]
- Levine R. A., Petokas S., Nandi J., Enthoven D. Effects of nonsteroidal, antiinflammatory drugs on gastrointestinal injury and prostanoid generation in healthy volunteers. Dig Dis Sci. 1988 Jun;33(6):660–666. doi: 10.1007/BF01540427. [DOI] [PubMed] [Google Scholar]
- Ligumsky M., Golanska E. M., Hansen D. G., Kauffman G. L., Jr Aspirin can inhibit gastric mucosal cyclo-oxygenase without causing lesions in rat. Gastroenterology. 1983 Apr;84(4):756–761. [PubMed] [Google Scholar]
- Lim S. G., Menzies I. S., Lee C. A., Johnson M. A., Pounder R. E. Intestinal permeability and function in patients infected with human immunodeficiency virus. A comparison with coeliac disease. Scand J Gastroenterol. 1993 Jul;28(7):573–580. doi: 10.3109/00365529309096090. [DOI] [PubMed] [Google Scholar]
- Maxton D. G., Bjarnason I., Reynolds A. P., Catt S. D., Peters T. J., Menzies I. S. Lactulose, 51Cr-labelled ethylenediaminetetra-acetate, L-rhamnose and polyethyleneglycol 400 [corrected] as probe markers for assessment in vivo of human intestinal permeability. Clin Sci (Lond) 1986 Jul;71(1):71–80. doi: 10.1042/cs0710071. [DOI] [PubMed] [Google Scholar]
- Melarange R., Gentry C., O'Connell C., Blower P. R., Neil C., Kelvin A. S., Toseland C. D. Antiinflammatory and gastrointestinal effects of nabumetone or its active metabolite, 6-methoxy-2-naphthylacetic acid (6MNA). Comparative studies with indomethacin. Dig Dis Sci. 1992 Dec;37(12):1847–1852. doi: 10.1007/BF01308078. [DOI] [PubMed] [Google Scholar]
- Meling T. R., Aabakken L., Røseth A., Osnes M. Faecal calprotectin shedding after short-term treatment with non-steroidal anti-inflammatory drugs. Scand J Gastroenterol. 1996 Apr;31(4):339–344. doi: 10.3109/00365529609006407. [DOI] [PubMed] [Google Scholar]
- Menzies I. S., Jenkins A. P., Heduan E., Catt S. D., Segal M. B., Creamer B. The effect of poorly absorbed solute on intestinal absorption. Scand J Gastroenterol. 1990 Dec;25(12):1257–1264. doi: 10.3109/00365529008998562. [DOI] [PubMed] [Google Scholar]
- Menzies I. S., Mount J. N., Wheeler M. J. Quantitative estimation of clinically important monosaccharides in plasma by rapid thin layer chromatography. Ann Clin Biochem. 1978 Mar;15(2):65–76. doi: 10.1177/000456327801500116. [DOI] [PubMed] [Google Scholar]
- Mitchell D. M., Spitz P. W., Young D. Y., Bloch D. A., McShane D. J., Fries J. F. Survival, prognosis, and causes of death in rheumatoid arthritis. Arthritis Rheum. 1986 Jun;29(6):706–714. doi: 10.1002/art.1780290602. [DOI] [PubMed] [Google Scholar]
- Morris A. J., Madhok R., Sturrock R. D., Capell H. A., MacKenzie J. F. Enteroscopic diagnosis of small bowel ulceration in patients receiving non-steroidal anti-inflammatory drugs. Lancet. 1991 Mar 2;337(8740):520–520. doi: 10.1016/0140-6736(91)91300-j. [DOI] [PubMed] [Google Scholar]
- Morris A. J., Wasson L. A., MacKenzie J. F. Small bowel enteroscopy in undiagnosed gastrointestinal blood loss. Gut. 1992 Jul;33(7):887–889. doi: 10.1136/gut.33.7.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Callahan L. F. Taking mortality in rheumatoid arthritis seriously--predictive markers, socioeconomic status and comorbidity. J Rheumatol. 1986 Oct;13(5):841–845. [PubMed] [Google Scholar]
- Quinn C. M., Bjarnason I., Price A. B. Gastritis in patients on non-steroidal anti-inflammatory drugs. Histopathology. 1993 Oct;23(4):341–348. doi: 10.1111/j.1365-2559.1993.tb01217.x. [DOI] [PubMed] [Google Scholar]
- Somasundaram S., Hayllar H., Rafi S., Wrigglesworth J. M., Macpherson A. J., Bjarnason I. The biochemical basis of non-steroidal anti-inflammatory drug-induced damage to the gastrointestinal tract: a review and a hypothesis. Scand J Gastroenterol. 1995 Apr;30(4):289–299. doi: 10.3109/00365529509093280. [DOI] [PubMed] [Google Scholar]
- Somasundaram S., Rafi S., Hayllar J., Sigthorsson G., Jacob M., Price A. B., Macpherson A., Mahmod T., Scott D., Wrigglesworth J. M. Mitochondrial damage: a possible mechanism of the "topical" phase of NSAID induced injury to the rat intestine. Gut. 1997 Sep;41(3):344–353. doi: 10.1136/gut.41.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teahon K., Smethurst P., Levi A. J., Menzies I. S., Bjarnason I. Intestinal permeability in patients with Crohn's disease and their first degree relatives. Gut. 1992 Mar;33(3):320–323. doi: 10.1136/gut.33.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis S., Menzies I. Intestinal permeability: functional assessment and significance. Clin Sci (Lond) 1992 May;82(5):471–488. doi: 10.1042/cs0820471. [DOI] [PubMed] [Google Scholar]
- Wallace J. L. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997 Mar;112(3):1000–1016. doi: 10.1053/gast.1997.v112.pm9041264. [DOI] [PubMed] [Google Scholar]