Abstract
Background—Germline mutations of the RET proto-oncogene identical to those found in the tumour predisposition syndrome multiple endocrine neoplasia type 2A (MEN2A), were detected in 2.5-5% of sporadic and familial cases of Hirschsprung's disease. Some patients with Hirschsprung's disease may therefore be exposed to a highly increased risk of tumours. Aims—To define clinical use of RET gene testing in Hirschsprung's disease and related patient management from an oncological point of view. Methods—Sixty patients with Hirschsprung's disease were screened for RET mutations. In three, MEN2A type RET mutations were detected. Case reports for these three patients are presented. Results and conclusions—Only 22 families or sporadic patients with Hirschsprung's disease and MEN2A type RET mutations have been reported. Therefore, it is difficult to predict tumour risk for patients with familial or sporadic Hirschsprung's disease, and their relatives, who carry these mutations. For these mutation carriers, periodic screening for tumours as in MEN2A is advised, but prophylactic thyroidectomy is offered hesitantly. RET gene testing in familial or sporadic Hirschsprung's disease is not recommended at present outside a complete clinical research setting. In combined MEN2A/Hirschsprung's disease families RET gene testing, tumour screening, and prophylactic thyroidectomy are indicated as in MEN2A.
Keywords: DNA analysis; Hirschsprung's disease; multiple endocrine neoplasia type 2A; RET
Full Text
The Full Text of this article is available as a PDF (115.3 KB).
Figure 1 .
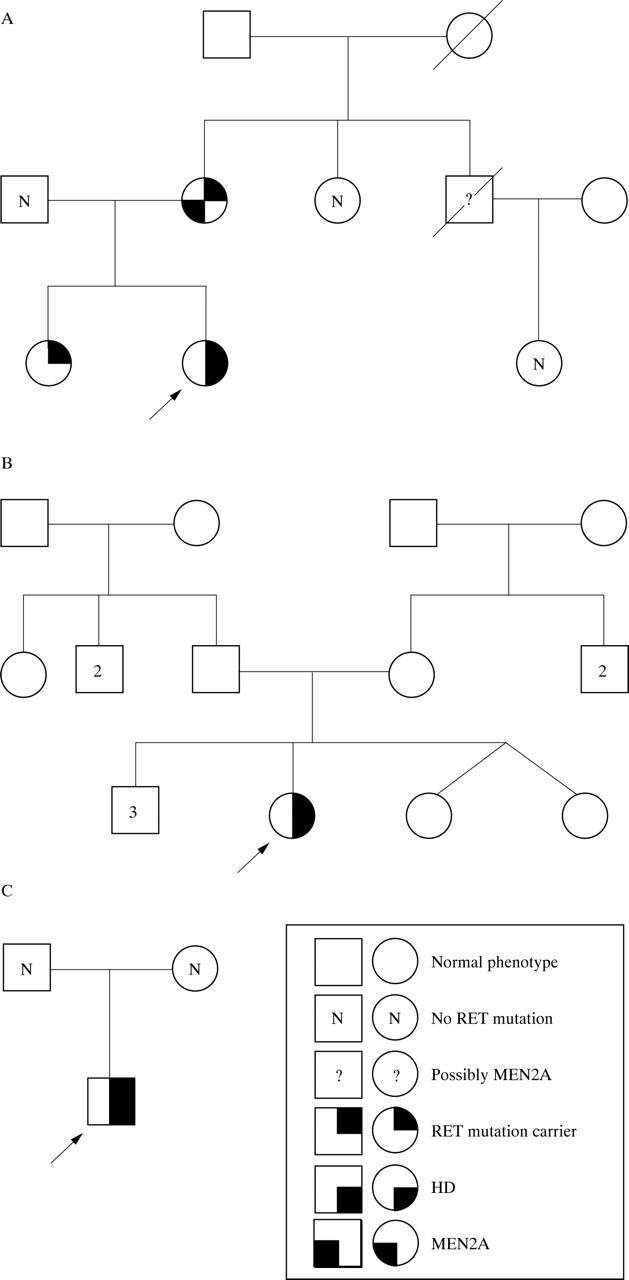
Pedigrees of the families of the index patients. In (A) patient 1 is indicated with an arrow. In (B) patient 2 is indicated with an arrow; she probably has combined MEN2A/HD. Her relatives could not be tested for the RET mutation or screened for MEN 2 tumours. Numbers within squares represent the additional number of asymptomatic males in that generation. In (C) patient 3 is indicated with an arrow; the mutation was not shown in his parents. HD, Hirschsprung's disease.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angrist M., Bolk S., Thiel B., Puffenberger E. G., Hofstra R. M., Buys C. H., Cass D. T., Chakravarti A. Mutation analysis of the RET receptor tyrosine kinase in Hirschsprung disease. Hum Mol Genet. 1995 May;4(5):821–830. doi: 10.1093/hmg/4.5.821. [DOI] [PubMed] [Google Scholar]
- Attie T., Pelet A., Sarda P., Eng C., Edery P., Mulligan L. M., Ponder B. A., Munnich A., Lyonnet S. A 7 bp deletion of the RET proto-oncogene in familial Hirschsprung's disease. Hum Mol Genet. 1994 Aug;3(8):1439–1440. doi: 10.1093/hmg/3.8.1439. [DOI] [PubMed] [Google Scholar]
- Attié T., Pelet A., Edery P., Eng C., Mulligan L. M., Amiel J., Boutrand L., Beldjord C., Nihoul-Fékété C., Munnich A. Diversity of RET proto-oncogene mutations in familial and sporadic Hirschsprung disease. Hum Mol Genet. 1995 Aug;4(8):1381–1386. doi: 10.1093/hmg/4.8.1381. [DOI] [PubMed] [Google Scholar]
- Badner J. A., Sieber W. K., Garver K. L., Chakravarti A. A genetic study of Hirschsprung disease. Am J Hum Genet. 1990 Mar;46(3):568–580. [PMC free article] [PubMed] [Google Scholar]
- Bergholm U., Bergström R., Ekbom A. Long-term follow-up of patients with medullary carcinoma of the thyroid. Cancer. 1997 Jan 1;79(1):132–138. doi: 10.1002/(sici)1097-0142(19970101)79:1<132::aid-cncr19>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Blank R. D., Sklar C. A., Dimich A. B., LaQuaglia M. P., Brennan M. F. Clinical presentations and RET protooncogene mutations in seven multiple endocrine neoplasia type 2 kindreds. Cancer. 1996 Nov 1;78(9):1996–2003. [PubMed] [Google Scholar]
- Borst M. J., VanCamp J. M., Peacock M. L., Decker R. A. Mutational analysis of multiple endocrine neoplasia type 2A associated with Hirschsprung's disease. Surgery. 1995 Apr;117(4):386–391. doi: 10.1016/s0039-6060(05)80057-1. [DOI] [PubMed] [Google Scholar]
- Buj-Bello A., Adu J., Piñn L. G., Horton A., Thompson J., Rosenthal A., Chinchetru M., Buchman V. L., Davies A. M. Neurturin responsiveness requires a GPI-linked receptor and the Ret receptor tyrosine kinase. Nature. 1997 Jun 12;387(6634):721–724. doi: 10.1038/42729. [DOI] [PubMed] [Google Scholar]
- Calmettes C., Ponder B. A., Fischer J. A., Raue F. Early diagnosis of the multiple endocrine neoplasia type 2 syndrome: consensus statement. European Community Concerted Action: Medullary Thyroid Carcinoma. Eur J Clin Invest. 1992 Nov;22(11):755–760. doi: 10.1111/j.1365-2362.1992.tb01441.x. [DOI] [PubMed] [Google Scholar]
- Carlomagno F., De Vita G., Berlingieri M. T., de Franciscis V., Melillo R. M., Colantuoni V., Kraus M. H., Di Fiore P. P., Fusco A., Santoro M. Molecular heterogeneity of RET loss of function in Hirschsprung's disease. EMBO J. 1996 Jun 3;15(11):2717–2725. [PMC free article] [PubMed] [Google Scholar]
- Caron P., Attié T., David D., Amiel J., Brousset F., Roger P., Munnich A., Lyonnet S. C618R mutation in exon 10 of the RET proto-oncogene in a kindred with multiple endocrine neoplasia type 2A and Hirschsprung's disease. J Clin Endocrinol Metab. 1996 Jul;81(7):2731–2733. doi: 10.1210/jcem.81.7.8675603. [DOI] [PubMed] [Google Scholar]
- Cosma M. P., Panariello L., Quadro L., Dathan N. A., Fattoruso O., Colantuoni V. A mutation in the RET proto-oncogene in Hirschsprung's disease affects the tyrosine kinase activity associated with multiple endocrine neoplasia type 2A and 2B. Biochem J. 1996 Mar 1;314(Pt 2):397–400. doi: 10.1042/bj3140397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker R. A. Long-term follow-up of a large North American kindred with multiple endocrine neoplasia type 2A. Surgery. 1992 Dec;112(6):1066–1073. [PubMed] [Google Scholar]
- Decker R. A., Peacock M. L., Watson P. Hirschsprung disease in MEN 2A: increased spectrum of RET exon 10 genotypes and strong genotype-phenotype correlation. Hum Mol Genet. 1998 Jan;7(1):129–134. doi: 10.1093/hmg/7.1.129. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H. The RET proto-oncogene and cancer. J Intern Med. 1995 Oct;238(4):319–325. doi: 10.1111/j.1365-2796.1995.tb01205.x. [DOI] [PubMed] [Google Scholar]
- Durbec P., Marcos-Gutierrez C. V., Kilkenny C., Grigoriou M., Wartiowaara K., Suvanto P., Smith D., Ponder B., Costantini F., Saarma M. GDNF signalling through the Ret receptor tyrosine kinase. Nature. 1996 Jun 27;381(6585):789–793. doi: 10.1038/381789a0. [DOI] [PubMed] [Google Scholar]
- Edery P., Lyonnet S., Mulligan L. M., Pelet A., Dow E., Abel L., Holder S., Nihoul-Fékété C., Ponder B. A., Munnich A. Mutations of the RET proto-oncogene in Hirschsprung's disease. Nature. 1994 Jan 27;367(6461):378–380. doi: 10.1038/367378a0. [DOI] [PubMed] [Google Scholar]
- Eng C., Clayton D., Schuffenecker I., Lenoir G., Cote G., Gagel R. F., van Amstel H. K., Lips C. J., Nishisho I., Takai S. I. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA. 1996 Nov 20;276(19):1575–1579. [PubMed] [Google Scholar]
- Gagel R. F., Cote G. J., Martins Bugalho M. J., Boyd A. E., 3rd, Cummings T., Goepfert H., Evans D. B., Cangir A., Khorana S., Schultz P. N. Clinical use of molecular information in the management of multiple endocrine neoplasia type 2A. J Intern Med. 1995 Oct;238(4):333–341. doi: 10.1111/j.1365-2796.1995.tb01207.x. [DOI] [PubMed] [Google Scholar]
- Gagel R. F., Tashjian A. H., Jr, Cummings T., Papathanasopoulos N., Kaplan M. M., DeLellis R. A., Wolfe H. J., Reichlin S. The clinical outcome of prospective screening for multiple endocrine neoplasia type 2a. An 18-year experience. N Engl J Med. 1988 Feb 25;318(8):478–484. doi: 10.1056/NEJM198802253180804. [DOI] [PubMed] [Google Scholar]
- Hofstra R. M., Sijmons R. H., Stelwagen T., Stulp R. P., Kousseff B. G., Lips C. J., Steijlen P. M., Van Voorst Vader P. C., Buys C. H. RET mutation screening in familial cutaneous lichen amyloidosis and in skin amyloidosis associated with multiple endocrine neoplasia. J Invest Dermatol. 1996 Aug;107(2):215–218. doi: 10.1111/1523-1747.ep12329651. [DOI] [PubMed] [Google Scholar]
- Ito S., Iwashita T., Asai N., Murakami H., Iwata Y., Sobue G., Takahashi M. Biological properties of Ret with cysteine mutations correlate with multiple endocrine neoplasia type 2A, familial medullary thyroid carcinoma, and Hirschsprung's disease phenotype. Cancer Res. 1997 Jul 15;57(14):2870–2872. [PubMed] [Google Scholar]
- Iwashita T., Murakami H., Asai N., Takahashi M. Mechanism of ret dysfunction by Hirschsprung mutations affecting its extracellular domain. Hum Mol Genet. 1996 Oct;5(10):1577–1580. doi: 10.1093/hmg/5.10.1577. [DOI] [PubMed] [Google Scholar]
- Klein R. D., Sherman D., Ho W. H., Stone D., Bennett G. L., Moffat B., Vandlen R., Simmons L., Gu Q., Hongo J. A. A GPI-linked protein that interacts with Ret to form a candidate neurturin receptor. Nature. 1997 Jun 12;387(6634):717–721. doi: 10.1038/42722. [DOI] [PubMed] [Google Scholar]
- Landsvater R. M., Rombouts A. G., te Meerman G. J., Schillhorn-van Veen J. M., Berends M. J., Geerdink R. A., Struyvenberg A., Buys C. H., Lips C. J. The clinical implications of a positive calcitonin test for C-cell hyperplasia in genetically unaffected members of an MEN2A kindred. Am J Hum Genet. 1993 Feb;52(2):335–342. [PMC free article] [PubMed] [Google Scholar]
- Ledger G. A., Khosla S., Lindor N. M., Thibodeau S. N., Gharib H. Genetic testing in the diagnosis and management of multiple endocrine neoplasia type II. Ann Intern Med. 1995 Jan 15;122(2):118–124. doi: 10.7326/0003-4819-122-2-199501150-00008. [DOI] [PubMed] [Google Scholar]
- Lips C. J., Landsvater R. M., Höppener J. W., Geerdink R. A., Blijham G., van Veen J. M., van Gils A. P., de Wit M. J., Zewald R. A., Berends M. J. Clinical screening as compared with DNA analysis in families with multiple endocrine neoplasia type 2A. N Engl J Med. 1994 Sep 29;331(13):828–835. doi: 10.1056/NEJM199409293311302. [DOI] [PubMed] [Google Scholar]
- Lyonnet S., Bolino A., Pelet A., Abel L., Nihoul-Fékété C., Briard M. L., Mok-Siu V., Kaariainen H., Martucciello G., Lerone M. A gene for Hirschsprung disease maps to the proximal long arm of chromosome 10. Nat Genet. 1993 Aug;4(4):346–350. doi: 10.1038/ng0893-346. [DOI] [PubMed] [Google Scholar]
- Marsh D. J., McDowall D., Hyland V. J., Andrew S. D., Schnitzler M., Gaskin E. L., Nevell D. F., Diamond T., Delbridge L., Clifton-Bligh P. The identification of false positive responses to the pentagastrin stimulation test in RET mutation negative members of MEN 2A families. Clin Endocrinol (Oxf) 1996 Feb;44(2):213–220. doi: 10.1046/j.1365-2265.1996.505292.x. [DOI] [PubMed] [Google Scholar]
- Mulligan L. M., Eng C., Attié T., Lyonnet S., Marsh D. J., Hyland V. J., Robinson B. G., Frilling A., Verellen-Dumoulin C., Safar A. Diverse phenotypes associated with exon 10 mutations of the RET proto-oncogene. Hum Mol Genet. 1994 Dec;3(12):2163–2167. doi: 10.1093/hmg/3.12.2163. [DOI] [PubMed] [Google Scholar]
- Mulligan L. M., Marsh D. J., Robinson B. G., Schuffenecker I., Zedenius J., Lips C. J., Gagel R. F., Takai S. I., Noll W. W., Fink M. Genotype-phenotype correlation in multiple endocrine neoplasia type 2: report of the International RET Mutation Consortium. J Intern Med. 1995 Oct;238(4):343–346. doi: 10.1111/j.1365-2796.1995.tb01208.x. [DOI] [PubMed] [Google Scholar]
- Pasini B., Borrello M. G., Greco A., Bongarzone I., Luo Y., Mondellini P., Alberti L., Miranda C., Arighi E., Bocciardi R. Loss of function effect of RET mutations causing Hirschsprung disease. Nat Genet. 1995 May;10(1):35–40. doi: 10.1038/ng0595-35. [DOI] [PubMed] [Google Scholar]
- Passarge E. The genetics of Hirschsprung's disease. Evidence for heterogeneous etiology and a study of sixty-three families. N Engl J Med. 1967 Jan 19;276(3):138–143. doi: 10.1056/NEJM196701192760303. [DOI] [PubMed] [Google Scholar]
- Pelet A., Attie T., Goulet O., Eng C., Ponder B. A., Munnich A., Lyonnet S. De-novo mutations of the RET proto-oncogene in Hirschsprung's disease. Lancet. 1994 Dec 24;344(8939-8940):1769–1770. doi: 10.1016/s0140-6736(94)92908-4. [DOI] [PubMed] [Google Scholar]
- Peretz H., Luboshitsky R., Baron E., Biton A., Gershoni R., Usher S., Grynberg E., Yakobson E., Graff E., Lapidot M. Cys 618 Arg mutation in the RET proto-oncogene associated with familial medullary thyroid carcinoma and maternally transmitted Hirschsprung's disease suggesting a role for imprinting. Hum Mutat. 1997;10(2):155–159. doi: 10.1002/(SICI)1098-1004(1997)10:2<155::AID-HUMU7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Ponder B. A., Ponder M. A., Coffey R., Pembrey M. E., Gagel R. F., Telenius-Berg M., Semple P., Easton D. F. Risk estimation and screening in families of patients with medullary thyroid carcinoma. Lancet. 1988 Feb 20;1(8582):397–401. doi: 10.1016/s0140-6736(88)91191-9. [DOI] [PubMed] [Google Scholar]
- Romeo G., Ronchetto P., Luo Y., Barone V., Seri M., Ceccherini I., Pasini B., Bocciardi R., Lerone M., Käriäinen H. Point mutations affecting the tyrosine kinase domain of the RET proto-oncogene in Hirschsprung's disease. Nature. 1994 Jan 27;367(6461):377–378. doi: 10.1038/367377a0. [DOI] [PubMed] [Google Scholar]
- Santoro M., Carlomagno F., Romano A., Bottaro D. P., Dathan N. A., Grieco M., Fusco A., Vecchio G., Matoskova B., Kraus M. H. Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science. 1995 Jan 20;267(5196):381–383. doi: 10.1126/science.7824936. [DOI] [PubMed] [Google Scholar]
- Schuchardt A., D'Agati V., Larsson-Blomberg L., Costantini F., Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994 Jan 27;367(6461):380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Seri M., Yin L., Barone V., Bolino A., Celli I., Bocciardi R., Pasini B., Ceccherini I., Lerone M., Kristoffersson U. Frequency of RET mutations in long- and short-segment Hirschsprung disease. Hum Mutat. 1997;9(3):243–249. doi: 10.1002/(SICI)1098-1004(1997)9:3<243::AID-HUMU5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Smith D. P., Eng C., Ponder B. A. Mutations of the RET proto-oncogene in the multiple endocrine neoplasia type 2 syndromes and Hirschsprung disease. J Cell Sci Suppl. 1994;18:43–49. doi: 10.1242/jcs.1994.supplement_18.6. [DOI] [PubMed] [Google Scholar]
- Trupp M., Arenas E., Fainzilber M., Nilsson A. S., Sieber B. A., Grigoriou M., Kilkenny C., Salazar-Grueso E., Pachnis V., Arumäe U. Functional receptor for GDNF encoded by the c-ret proto-oncogene. Nature. 1996 Jun 27;381(6585):785–789. doi: 10.1038/381785a0. [DOI] [PubMed] [Google Scholar]
- Wells S. A., Jr, Chi D. D., Toshima K., Dehner L. P., Coffin C. M., Dowton S. B., Ivanovich J. L., DeBenedetti M. K., Dilley W. G., Moley J. F. Predictive DNA testing and prophylactic thyroidectomy in patients at risk for multiple endocrine neoplasia type 2A. Ann Surg. 1994 Sep;220(3):237–250. doi: 10.1097/00000658-199409000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohllk N., Cote G. J., Evans D. B., Goepfert H., Ordonez N. G., Gagel R. F. Application of genetic screening information to the management of medullary thyroid carcinoma and multiple endocrine neoplasia type 2. Endocrinol Metab Clin North Am. 1996 Mar;25(1):1–25. doi: 10.1016/s0889-8529(05)70310-8. [DOI] [PubMed] [Google Scholar]
- Yin L., Barone V., Seri M., Bolino A., Bocciardi R., Ceccherini I., Pasini B., Tocco T., Lerone M., Cywes S. Heterogeneity and low detection rate of RET mutations in Hirschsprung disease. Eur J Hum Genet. 1994;2(4):272–280. doi: 10.1159/000472371. [DOI] [PubMed] [Google Scholar]


