Abstract
Background—The functions of urokinase in intestinal epithelia are unknown. Aims—To determine the relation of urokinase expressed by intestinal epithelial cells to their position in the crypt-villus/surface axis and of mucosal urokinase activity to epithelial proliferative kinetics in the distal colon. Methods—Urokinase expression was examined immunohistochemically in human intestinal mucosa. Urokinase activity was measured colorimetrically in epithelial cells isolated sequentially from the crypt-villus axis of the rat small intestine. In separate experiments, urokinase activity and epithelial kinetics (measured stathmokinetically) were measured in homogenates of distal colonic mucosa of 14 groups of eight rats fed diets known to alter epithelial turnover. Results—From the crypt base, an ascending gradient of expression and activity of urokinase was associated with the epithelial cells. Median mucosal urokinase activities in each of the dietary groups of rats correlated positively with autologous median number of metaphase arrests per crypt (r=0.68; p<0.005) and per 100 crypt cells (r=0.75; p<0.001), but not with crypt column height. Conclusions—Localisation of an enzyme capable of leading to digestion of cell substratum in the region where cells are loosely attached to their basement membrane, and the association of its activity with indexes of cell turnover, suggest a role for urokinase in facilitating epithelial cell loss in the intestine.
Keywords: urokinase; intestinal epithelium; colon; epithelial proliferation
Full Text
The Full Text of this article is available as a PDF (224.6 KB).
Figure 1 .
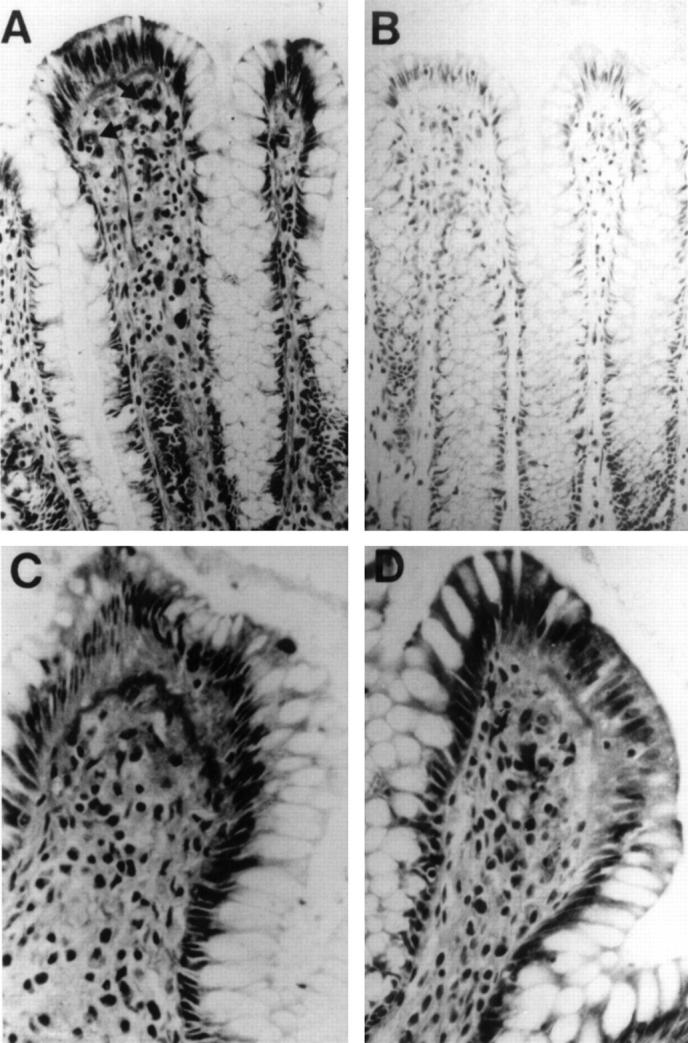
Immunohistochemical staining of sections of histologically normal colonic mucosa from patients with colorectal cancer. (A) Urokinase staining of upper crypt and surface epithelium (original magnification ×400). Arrows show specific staining of cells with morphology consistent with macrophages. (B) Negative control for (A). (D) and (C) Higher power magnification (original magnification ×800) of colonic sections from two other patients showing staining of cytoplasm of surface colonic epithelial cells, and of the basal and underlying basement membranes.
Figure 2 .
Immunohistochemical staining for urokinase of a section of normal human duodenal mucosa. (A) High power magnification (original magnification ×800) of the tip of a duodenal villus showing cytoplasmic staining for urokinase. Enterocytes in the lower villus and crypt showed minimal staining for urokinase (not shown). (B) Negative control of (A).
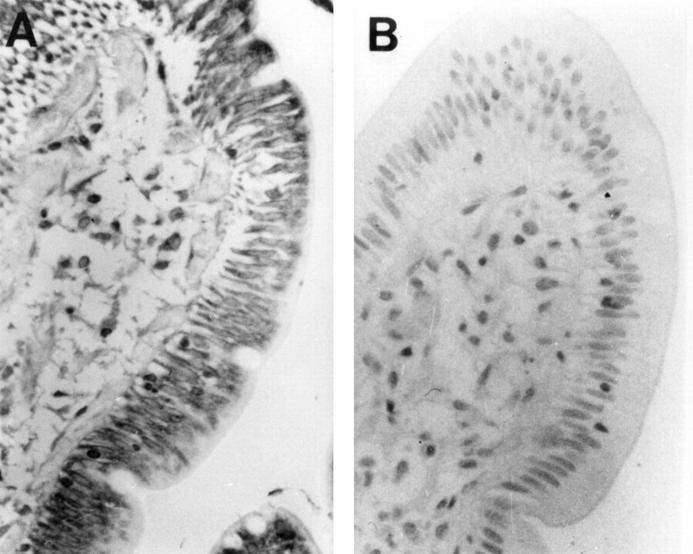
Figure 3 .
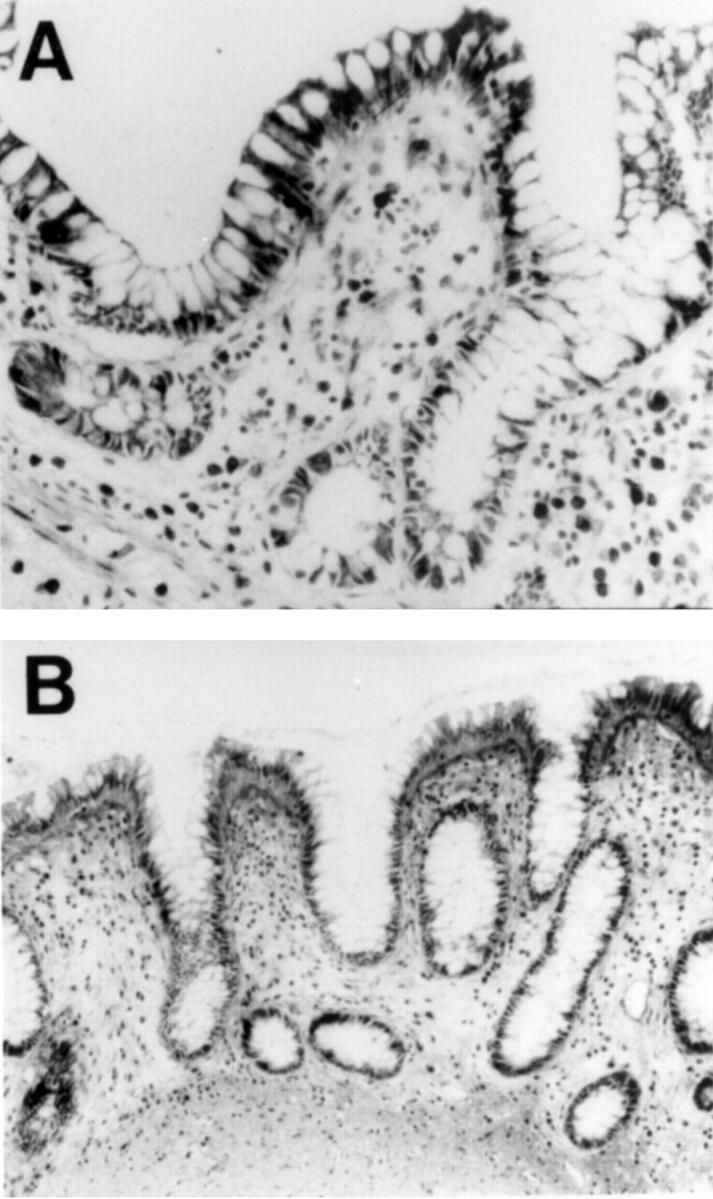
Immunohistochemical staining for urokinase in terminal ileum and colon affected by inflammatory bowel disease. (A) Terminal ileum of a patient with Crohn's disease showing cytoplasmic staining of villous but not crypt epithelium (original magnification ×400). (B) Colonic mucosa of a patient with ulcerative colitis, showing distorted crypt architecture but heavy staining of surface epithelial cells and underlying basement membrane (original magnification ×400).
Figure 4 .
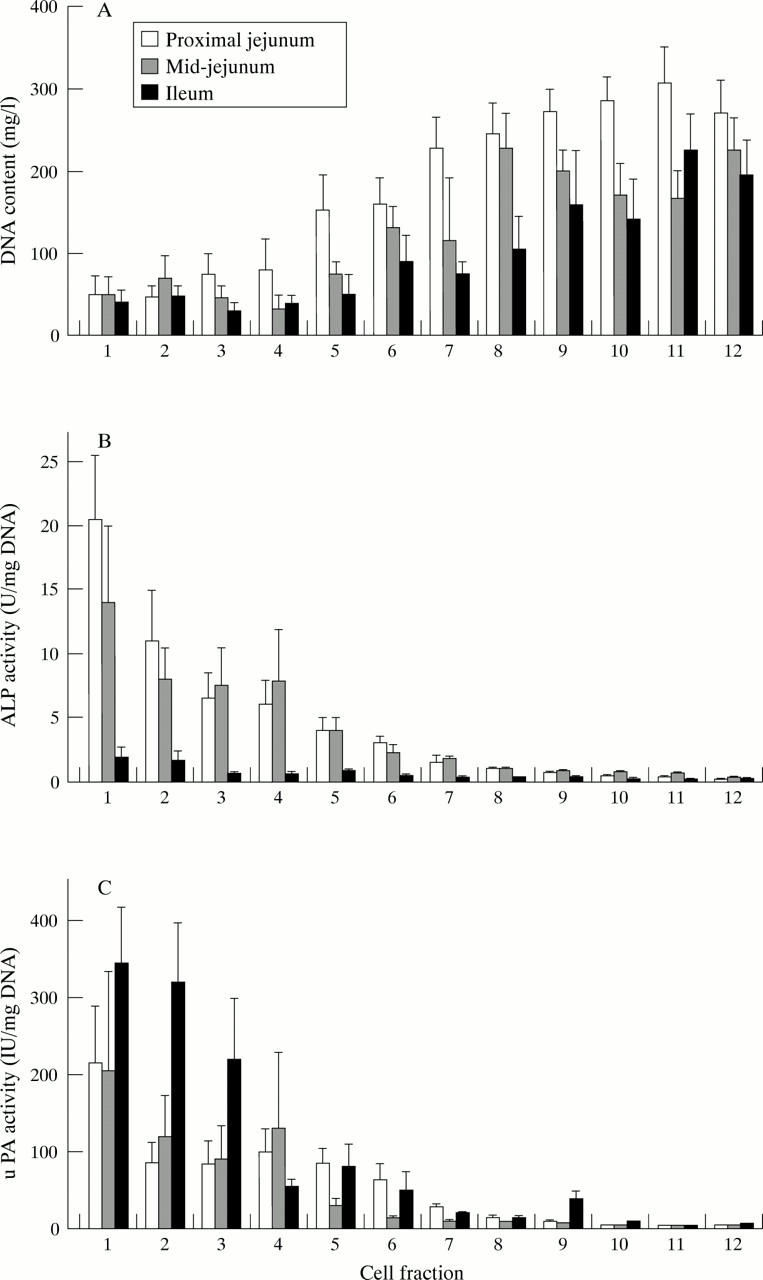
(A) DNA content, (B) alkaline phosphatase activities (ALP), and (C) urokinase (uPA) activities in enterocyte cell fractions from proximal jejunum, mid-jejunum, and ileum of normal rats. Results are shown as mean (SEM). Statistically significant differences (ANOVA) were found across cell fractions for DNA content (all p<0.001), alkaline phosphatase activities (p<0.001 for proximal and mid-jejunum, p=0.009 for ileum), and urokinase activities for proximal jejunum and ileum only (p<0.001 for both).
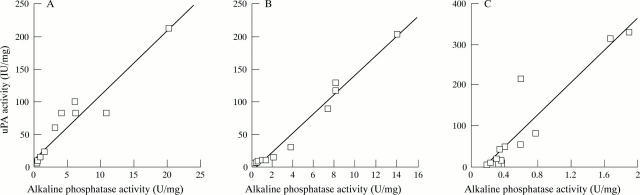
Figure 5 .
Comparison of mean alkaline phosphatase activities with mean urokinase (uPA) activities across 12 cell fractions from (A) proximal jejunum (r=0.95), (B) mid-jejunum (r=0.99), and (C) ileum (r=0.93). Correlations were all highly significant (p<0.0001; linear regression analysis).
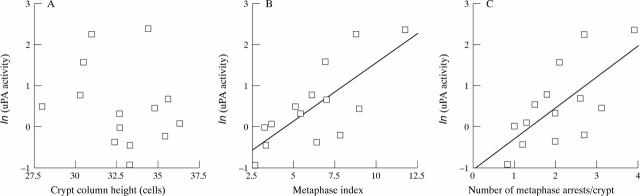
Figure 6 .
Correlation of (A) median crypt column height, (B) metaphase index, and (C) number of metaphase arrests per crypt column of distal colonic epithelium with median uPA activities (logarithm transformed) in homogenates of adjacent colonic mucosa in normal rats ingesting a variety of diets. Pearson correlation coefficients were −0.25 for crypt column height (p>0.1), 0.68 (p<0.005) for the number of arrests per crypt, and 0.75 (p<0.001) for the metaphase index.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert V., Young G. P., Morton C. L., Robinson P., Bhathal P. S. Systemic factors are trophic in bypassed rat small intestine in the absence of luminal contents. Gut. 1990 Mar;31(3):311–316. doi: 10.1136/gut.31.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates R. C., Buret A., van Helden D. F., Horton M. A., Burns G. F. Apoptosis induced by inhibition of intercellular contact. J Cell Biol. 1994 Apr;125(2):403–415. doi: 10.1083/jcb.125.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buck R. C. Ultrastructural features of rectal epithelium of the mouse during the early phases of migration to repair a defect. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;51(4):331–340. doi: 10.1007/BF02899042. [DOI] [PubMed] [Google Scholar]
- Buø L., Lyberg T., Jørgensen L., Johansen H. T., Aasen A. O. Location of plasminogen activator (PA) and PA inhibitor in human colorectal adenocarcinomas. APMIS. 1993 Mar;101(3):235–241. doi: 10.1111/j.1699-0463.1993.tb00106.x. [DOI] [PubMed] [Google Scholar]
- Cameron I. L., Ord V. A., Hunter K. E., Padilla G. M., Heitman D. W. Suppression of a a carcinogen (1,2-dimethylhydrazine dihydrochloride)-induced increase in mitotic activity in the colonic crypts of rats by addition of dietary cellulose. Cancer Res. 1989 Feb 15;49(4):991–995. [PubMed] [Google Scholar]
- Coleman P. L., Green G. D. A sensitive, coupled assay for plasminogen activator using a thiol ester substrate for plasmin. Ann N Y Acad Sci. 1981;370:617–626. doi: 10.1111/j.1749-6632.1981.tb29768.x. [DOI] [PubMed] [Google Scholar]
- Folino M., McIntyre A., Young G. P. Dietary fibers differ in their effects on large bowel epithelial proliferation and fecal fermentation-dependent events in rats. J Nutr. 1995 Jun;125(6):1521–1528. doi: 10.1093/jn/125.6.1521. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson P. R., Rosella O., Rosella G., Young G. P. Butyrate is a potent inhibitor of urokinase secretion by normal colonic epithelium in vitro. Gastroenterology. 1994 Aug;107(2):410–419. doi: 10.1016/0016-5085(94)90166-x. [DOI] [PubMed] [Google Scholar]
- Gibson P. R., van de Pol E., Doe W. F. Cell associated urokinase activity and colonic epithelial cells in health and disease. Gut. 1991 Feb;32(2):191–195. doi: 10.1136/gut.32.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson P., Folino M., McIntyre A., Rosella O., Finch C., Young G. Dietary modulation of colonic mucosal urokinase activity in rats. J Gastroenterol Hepatol. 1995 May-Jun;10(3):324–330. doi: 10.1111/j.1440-1746.1995.tb01101.x. [DOI] [PubMed] [Google Scholar]
- Gibson P., Rosella O., Rosella G., Young G. Secretion of urokinase and plasminogen activator inhibitor-1 by normal colonic epithelium in vitro. Gut. 1994 Jul;35(7):969–975. doi: 10.1136/gut.35.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson P., Rosella O., Young G. Serum free medium increases expression of markers of differentiation in human colonic crypt cells. Gut. 1994 Jun;35(6):791–797. doi: 10.1136/gut.35.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. I., Schwimmer R., Quigley J. P. Human plasma fibronectin as a substrate for human urokinase. Biochem J. 1989 Sep 1;262(2):529–534. doi: 10.1042/bj2620529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratecos D., Knibiehler M., Benoit V., Sémériva M. Plasma membranes from rat intestinal epithelial cells at different stages of maturation. I. Preparation and characterization of plasma membrane subfractions originating from crypt cells and from villous cells. Biochim Biophys Acta. 1978 Oct 4;512(3):508–524. doi: 10.1016/0005-2736(78)90161-x. [DOI] [PubMed] [Google Scholar]
- Grøndahl-Hansen J., Agerlin N., Munkholm-Larsen P., Bach F., Nielsen L. S., Dombernowsky P., Danø K. Sensitive and specific enzyme-linked immunosorbent assay for urokinase-type plasminogen activator and its application to plasma from patients with breast cancer. J Lab Clin Med. 1988 Jan;111(1):42–51. [PubMed] [Google Scholar]
- Grøndahl-Hansen J., Ralfkiaer E., Kirkeby L. T., Kristensen P., Lund L. R., Danø K. Localization of urokinase-type plasminogen activator in stromal cells in adenocarcinomas of the colon in humans. Am J Pathol. 1991 Jan;138(1):111–117. [PMC free article] [PubMed] [Google Scholar]
- Hart D. A., Rehemtulla A. Plasminogen activators and their inhibitors: regulators of extracellular proteolysis and cell function. Comp Biochem Physiol B. 1988;90(4):691–708. doi: 10.1016/0305-0491(88)90323-9. [DOI] [PubMed] [Google Scholar]
- Hermiston M. L., Gordon J. I. In vivo analysis of cadherin function in the mouse intestinal epithelium: essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. J Cell Biol. 1995 Apr;129(2):489–506. doi: 10.1083/jcb.129.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollas W., Boyd D. Urokinase-dependent proteolysis in cultured colon cancer is directed by its receptor. Semin Thromb Hemost. 1991 Jul;17(3):225–230. doi: 10.1055/s-2007-1002613. [DOI] [PubMed] [Google Scholar]
- Kirchheimer J. C., Binder B. R. Function of receptor-bound urokinase. Semin Thromb Hemost. 1991 Jul;17(3):246–250. doi: 10.1055/s-2007-1002616. [DOI] [PubMed] [Google Scholar]
- Kohga S., Harvey S. R., Weaver R. M., Markus G. Localization of plasminogen activators in human colon cancer by immunoperoxidase staining. Cancer Res. 1985 Apr;45(4):1787–1796. [PubMed] [Google Scholar]
- Koretz K., Möller P., Schwartz-Albiez R. Plasminogen activators and plasminogen activator inhibitors in human colorectal carcinoma tissues are not expressed by the tumour cells. Eur J Cancer. 1993;29A(8):1184–1189. doi: 10.1016/s0959-8049(05)80312-0. [DOI] [PubMed] [Google Scholar]
- Kristensen P., Eriksen J., Danø K. Localization of urokinase-type plasminogen activator messenger RNA in the normal mouse by in situ hybridization. J Histochem Cytochem. 1991 Mar;39(3):341–349. doi: 10.1177/39.3.1899685. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lazarus G. S., Jensen P. J. Plasminogen activators in epithelial biology. Semin Thromb Hemost. 1991 Jul;17(3):210–216. doi: 10.1055/s-2007-1002611. [DOI] [PubMed] [Google Scholar]
- McIntyre A., Young G. P., Taranto T., Gibson P. R., Ward P. B. Different fibers have different regional effects on luminal contents of rat colon. Gastroenterology. 1991 Nov;101(5):1274–1281. doi: 10.1016/0016-5085(91)90077-x. [DOI] [PubMed] [Google Scholar]
- Morimoto K., Mishima H., Nishida T., Otori T. Role of urokinase type plasminogen activator (u-PA) in corneal epithelial migration. Thromb Haemost. 1993 Apr 1;69(4):387–391. [PubMed] [Google Scholar]
- Pyke C., Kristensen P., Ralfkiaer E., Grøndahl-Hansen J., Eriksen J., Blasi F., Danø K. Urokinase-type plasminogen activator is expressed in stromal cells and its receptor in cancer cells at invasive foci in human colon adenocarcinomas. Am J Pathol. 1991 May;138(5):1059–1067. [PMC free article] [PubMed] [Google Scholar]
- Pöllänen J., Saksela O., Salonen E. M., Andreasen P., Nielsen L., Danø K., Vaheri A. Distinct localizations of urokinase-type plasminogen activator and its type 1 inhibitor under cultured human fibroblasts and sarcoma cells. J Cell Biol. 1987 Apr;104(4):1085–1096. doi: 10.1083/jcb.104.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M., Chucholowski N., Busch E., Hellmann D., Wagner B., Goretzki L., Jänicke F., Günzler W. A., Graeff H. Fluorescent probes as tools to assess the receptor for the urokinase-type plasminogen activator on tumor cells. Semin Thromb Hemost. 1991 Jul;17(3):291–302. doi: 10.1055/s-2007-1002623. [DOI] [PubMed] [Google Scholar]
- Serafini E. P., Kirk A. P., Chambers T. J. Rate and pattern of epithelial cell proliferation in ulcerative colitis. Gut. 1981 Aug;22(8):648–652. doi: 10.1136/gut.22.8.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sier C. F., Fellbaum C., Verspaget H. W., Schmitt M., Griffioen G., Graeff H., Hôfler H., Lamers C. B. Immunolocalization of urokinase-type plasminogen activator in adenomas and carcinomas of the colorectum. Histopathology. 1991 Sep;19(3):231–237. doi: 10.1111/j.1365-2559.1991.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Sim P. S., Stephens R. W., Fayle D. R., Doe W. F. Urokinase-type plasminogen activator in colorectal carcinomas and adenomatous polyps: quantitative expression of active and proenzyme. Int J Cancer. 1988 Oct 15;42(4):483–488. doi: 10.1002/ijc.2910420402. [DOI] [PubMed] [Google Scholar]
- Stephens R. W., Aumailley M., Timpl R., Reisberg T., Tapiovaara H., Myöhänen H., Murphy-Ullrich J., Vaheri A. Urokinase binding to laminin-nidogen. Structural requirements and interactions with heparin. Eur J Biochem. 1992 Aug 1;207(3):937–942. doi: 10.1111/j.1432-1033.1992.tb17127.x. [DOI] [PubMed] [Google Scholar]
- Stephens R. W., Pöllänen J., Tapiovaara H., Leung K. C., Sim P. S., Salonen E. M., Rønne E., Behrendt N., Danø K., Vaheri A. Activation of pro-urokinase and plasminogen on human sarcoma cells: a proteolytic system with surface-bound reactants. J Cell Biol. 1989 May;108(5):1987–1995. doi: 10.1083/jcb.108.5.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sträter J., Wedding U., Barth T. F., Koretz K., Elsing C., Möller P. Rapid onset of apoptosis in vitro follows disruption of beta 1-integrin/matrix interactions in human colonic crypt cells. Gastroenterology. 1996 Jun;110(6):1776–1784. doi: 10.1053/gast.1996.v110.pm8964403. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Ikeo K., Gojobori T., Tanifuji M. Local function of urokinase receptor at the adhesion contact sites of a metastatic tumor cell. Thromb Res Suppl. 1990;10:55–61. doi: 10.1016/0049-3848(90)90378-p. [DOI] [PubMed] [Google Scholar]
- Tanaka N., Fukao H., Ueshima S., Okada K., Yasutomi M., Matsuo O. Plasminogen activator inhibitor 1 in human carcinoma tissues. Int J Cancer. 1991 Jun 19;48(4):481–484. doi: 10.1002/ijc.2910480402. [DOI] [PubMed] [Google Scholar]
- Terpstra O. T., van Blankenstein M., Dees J., Eilers G. A. Abnormal pattern of cell proliferation in the entire colonic mucosa of patients with colon adenoma or cancer. Gastroenterology. 1987 Mar;92(3):704–708. doi: 10.1016/0016-5085(87)90021-7. [DOI] [PubMed] [Google Scholar]
- Wei Y., Lukashev M., Simon D. I., Bodary S. C., Rosenberg S., Doyle M. V., Chapman H. A. Regulation of integrin function by the urokinase receptor. Science. 1996 Sep 13;273(5281):1551–1555. doi: 10.1126/science.273.5281.1551. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Young G. P., McIntyre A., Albert V., Folino M., Muir J. G., Gibson P. R. Wheat bran suppresses potato starch--potentiated colorectal tumorigenesis at the aberrant crypt stage in a rat model. Gastroenterology. 1996 Feb;110(2):508–514. doi: 10.1053/gast.1996.v110.pm8566598. [DOI] [PubMed] [Google Scholar]
- Young G. P., Rose I. S., Cropper S., Seetharam S., Alpers D. H. Hepatic clearance of rat plasma intestinal alkaline phosphatase. Am J Physiol. 1984 Oct;247(4 Pt 1):G419–G426. doi: 10.1152/ajpgi.1984.247.4.G419. [DOI] [PubMed] [Google Scholar]