Abstract
Background/Aim—Interleukin (IL) 12 is involved in the mucosal response during intestinal inflammation but its role is not fully understood. The response of human lamina propria T lymphocytes (T-LPL) to IL-12 in terms of interferon γ (IFN-γ) release and proliferation was investigated, exploring whether IL-15 and IL-7 cooperate with IL-12. The role of accessory molecules (CD2 and CD28) was also investigated. Methods—Unstimulated and phytohaemagglutinin preactivated T-LPL cultures were incubated with or without the initial addition of cytokines, anti-CD2 or anti-CD28 antibodies. IFN-γ mRNA was detected by reverse transcriptase polymerase chain reaction, and protein secretion was measured by enzyme linked immunosorbent assay (ELISA). Results—IFN-γ mRNA was induced in T-LPLs by IL-12 and IL-15 but not IL-7, whereas IFN-γ was measured only in IL-12 stimulated T-LPL cultures. IL-12 induced IFN-γ release was not abrogated by neutralising anti-IL-2 antibody or by cyclosporin A. IL-12 synergised with either anti-CD2 or anti-CD28 antibodies in inducing IFN-γ synthesis. In preactivated T-LPLs, IL-7 enhanced IFN-γ release induced by both IL-12 and anti-CD2, whereas IL-15 potentiated only IL-12 induced IFN-γ synthesis. IL-12 did not induce proliferation of either unstimulated or preactivated T-LPLs and it did not enhance the CD2/CD28 stimulated T-LPL proliferative response. No transcript for IL-12 receptor β1 subunit was detected in freshly isolated and activated T-LPLs whereas the β2 subunit mRNA was consistently found in T-LPL samples. Conclusions—IL-12 induces human T-LPLs to produce and release IFN-γ, and IL-15 and IL-7 cooperate with IL-12 in expanding the IFN-γ mucosal response.
Keywords: interleukin 12; interleukin 7; interleukin 15; interferon γ; intestinal inflammation
Full Text
The Full Text of this article is available as a PDF (169.1 KB).
Figure 1 .
Interferon γ (IFN-γ) release in three day cultures of both interleukin (IL) 12- and IL-12 (1000 pg/ml final concentration) plus anti-IL-12 antibody-stimulated lamina propria T lymphocytes. Each point on the curve represents the mean of all experiments; vertical bars indicate 1 SD. At each time interval, IL-12 induced IFN-γ release in a dose dependent manner (p = 0.01).
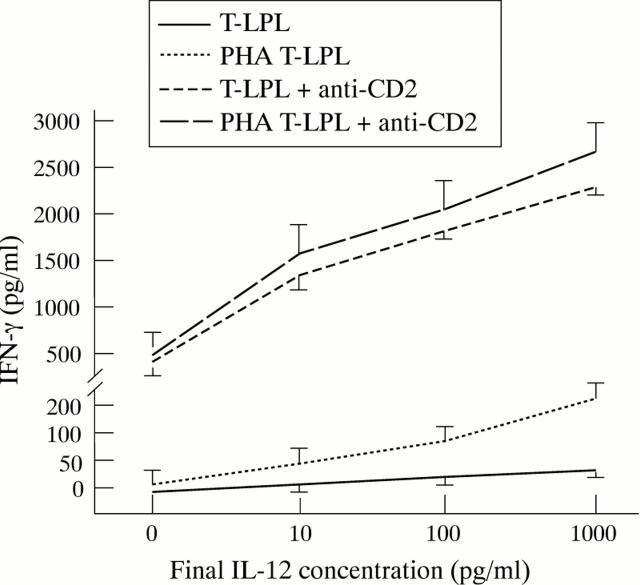
Figure 2 .
Interferon γ (IFN-γ) release in both lamina propria T lymphocytes (T-LPLs) and phytohaemagglutinin (PHA) preactivated T-LPLs after 24 hours of culture. Both T-LPLs and PHA preactivated T-LPLs were incubated with graded doses of interleukin (IL) 12 in the presence or absence of anti-CD2 antibody. Each point on the curve represents the mean of six representative experiments; vertical bars indicate 1 SD. Values of IFN-γ measured after co-stimulation with IL-12 and anti-CD2 antibody were significantly higher than observed after stimulation with IL-12 (p<0.0001) or anti-CD2 antibody alone (p<0.0001).
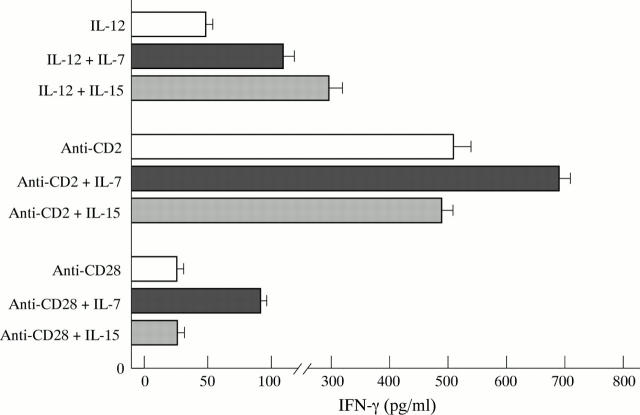
Figure 3 .
Interferon γ (IFN-γ) release by phytohaemagglutinin preactivated lamina propria T lymphocytes (T-LPLs) after 24 hours of culture. Cells were cultured with interleukin (IL) 12 (100 pg/ml final concentration) or anti-CD28 (1 µg/ml final concentration) or anti-CD2 (1:1000 final dilution) antibody in the presence or absence of IL-7 (10 ng/ml final concentration) or IL-15 (10 ng/ml final concentration). Data are the mean of six representative experiments; horizontal bars indicate 1 SD. The co-stimulation with IL-7 significantly enhanced T-LPL IFN-γ release induced by IL-12 (p<0.0001) or anti-CD28 (p<0.0001) or CD2 (p<0.0001) antibody. IL-15 potentiated IL-12 stimulated T-LPL IFN-γ release (p<0.0001) but not that induced by anti-CD28 or CD2 antibody.
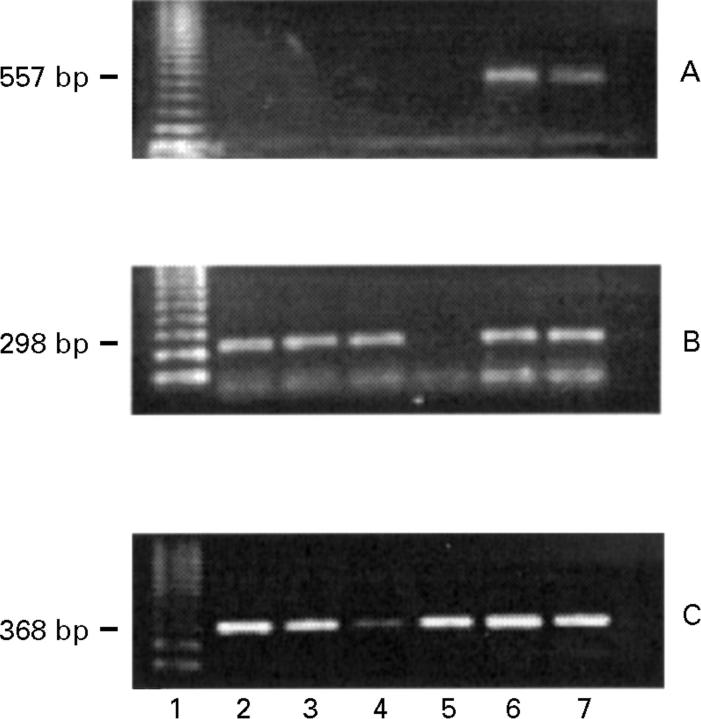
Figure 4 .
Agarose gel stained with ethidium bromide showing RT-PCR products for IL-12Rβ1 (557 bp; A) (after 35 cycles), IL-12Rβ2 (298 bp; B) (after 35 cycles), and glyceraldehyde-3-phosphate dehydrogenase (368 bp; C) (after 22 cycles) in lamina propria T lymphocytes (T-LPLs) and autologous peripheral blood T lymphocytes (T-PBLs) cultured with or without stimulation. Lane 1, 123 bp ladder; lane 2, unstimulated T-LPLs; lane 3, PHA (1µg/ml) stimulated T-LPLs; lane 4, anti-CD2/anti-CD28 antibody stimulated T-LPLs; lane 5, unstimulated T-PBLs; lane 6, PHA (1µg/ml) stimulated T-PBLs; lane 7, anti-CD2/anti-CD28 antibody stimulated T-PBLs.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilenker M., Roberts A. I., Brolin R. E., Ebert E. C. Interleukin-7 activates intestinal lymphocytes. Dig Dis Sci. 1995 Aug;40(8):1744–1749. doi: 10.1007/BF02212696. [DOI] [PubMed] [Google Scholar]
- Borger P., Kauffman H. F., Postma D. S., Vellenga E. IL-7 differentially modulates the expression of IFN-gamma and IL-4 in activated human T lymphocytes by transcriptional and post-transcriptional mechanisms. J Immunol. 1996 Feb 15;156(4):1333–1338. [PubMed] [Google Scholar]
- Boussiotis V. A., Barber D. L., Nakarai T., Freeman G. J., Gribben J. G., Bernstein G. M., D'Andrea A. D., Ritz J., Nadler L. M. Prevention of T cell anergy by signaling through the gamma c chain of the IL-2 receptor. Science. 1994 Nov 11;266(5187):1039–1042. doi: 10.1126/science.7973657. [DOI] [PubMed] [Google Scholar]
- Breese E., Braegger C. P., Corrigan C. J., Walker-Smith J. A., MacDonald T. T. Interleukin-2- and interferon-gamma-secreting T cells in normal and diseased human intestinal mucosa. Immunology. 1993 Jan;78(1):127–131. [PMC free article] [PubMed] [Google Scholar]
- Caligiuri M. A., Zmuidzinas A., Manley T. J., Levine H., Smith K. A., Ritz J. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J Exp Med. 1990 May 1;171(5):1509–1526. doi: 10.1084/jem.171.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Croft M., Carter L., Swain S. L., Dutton R. W. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994 Nov 1;180(5):1715–1728. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maria R., Fais S., Silvestri M., Frati L., Pallone F., Santoni A., Testi R. Continuous in vivo activation and transient hyporesponsiveness to TcR/CD3 triggering of human gut lamina propria lymphocytes. Eur J Immunol. 1993 Dec;23(12):3104–3108. doi: 10.1002/eji.1830231209. [DOI] [PubMed] [Google Scholar]
- Fais S., Capobianchi M. R., Pallone F., Di Marco P., Boirivant M., Dianzani F., Torsoli A. Spontaneous release of interferon gamma by intestinal lamina propria lymphocytes in Crohn's disease. Kinetics of in vitro response to interferon gamma inducers. Gut. 1991 Apr;32(4):403–407. doi: 10.1136/gut.32.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar M. A., Schreiber R. D. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- Fuss I. J., Neurath M., Boirivant M., Klein J. S., de la Motte C., Strong S. A., Fiocchi C., Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996 Aug 1;157(3):1261–1270. [PubMed] [Google Scholar]
- Gately M. K., Desai B. B., Wolitzky A. G., Quinn P. M., Dwyer C. M., Podlaski F. J., Familletti P. C., Sinigaglia F., Chizonnite R., Gubler U. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor). J Immunol. 1991 Aug 1;147(3):874–882. [PubMed] [Google Scholar]
- Gollob J. A., Li J., Reinherz E. L., Ritz J. CD2 regulates responsiveness of activated T cells to interleukin 12. J Exp Med. 1995 Sep 1;182(3):721–731. doi: 10.1084/jem.182.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubin M., Kamoun M., Trinchieri G. Interleukin 12 synergizes with B7/CD28 interaction in inducing efficient proliferation and cytokine production of human T cells. J Exp Med. 1994 Jul 1;180(1):211–222. doi: 10.1084/jem.180.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Chow J. M., Gri G., Carra G., Gerosa F., Wolf S. F., Dzialo R., Trinchieri G. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J Exp Med. 1996 Jan 1;183(1):147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott R. P., Stenson W. F. Alterations of the immune system in ulcerative colitis and Crohn's disease. Adv Immunol. 1988;42:285–328. doi: 10.1016/s0065-2776(08)60848-2. [DOI] [PubMed] [Google Scholar]
- Magram J., Connaughton S. E., Warrier R. R., Carvajal D. M., Wu C. Y., Ferrante J., Stewart C., Sarmiento U., Faherty D. A., Gately M. K. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996 May;4(5):471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- McInnes I. B., Leung B. P., Sturrock R. D., Field M., Liew F. Y. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis. Nat Med. 1997 Feb;3(2):189–195. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- McInnes I. B., al-Mughales J., Field M., Leung B. P., Huang F. P., Dixon R., Sturrock R. D., Wilkinson P. C., Liew F. Y. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med. 1996 Feb;2(2):175–182. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- Mehrotra P. T., Grant A. J., Siegel J. P. Synergistic effects of IL-7 and IL-12 on human T cell activation. J Immunol. 1995 May 15;154(10):5093–5102. [PubMed] [Google Scholar]
- Metcalf D. Hematopoietic regulators: redundancy or subtlety? Blood. 1993 Dec 15;82(12):3515–3523. [PubMed] [Google Scholar]
- Monteleone G., Biancone L., Marasco R., Morrone G., Marasco O., Luzza F., Pallone F. Interleukin 12 is expressed and actively released by Crohn's disease intestinal lamina propria mononuclear cells. Gastroenterology. 1997 Apr;112(4):1169–1178. doi: 10.1016/s0016-5085(97)70128-8. [DOI] [PubMed] [Google Scholar]
- Neurath M. F., Fuss I., Kelsall B. L., Stüber E., Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995 Nov 1;182(5):1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallone F., Fais S., Squarcia O., Biancone L., Pozzilli P., Boirivant M. Activation of peripheral blood and intestinal lamina propria lymphocytes in Crohn's disease. In vivo state of activation and in vitro response to stimulation as defined by the expression of early activation antigens. Gut. 1987 Jun;28(6):745–753. doi: 10.1136/gut.28.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallone F., Montano S., Fais S., Boirivant M., Signore A., Pozzilli P. Studies of peripheral blood lymphocytes in Crohn's disease. Circulating activated T cells. Scand J Gastroenterol. 1983 Nov;18(8):1003–1008. doi: 10.3109/00365528309181833. [DOI] [PubMed] [Google Scholar]
- Presky D. H., Yang H., Minetti L. J., Chua A. O., Nabavi N., Wu C. Y., Gately M. K., Gubler U. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc Natl Acad Sci U S A. 1996 Nov 26;93(24):14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quill H., Bhandoola A., Trinchieri G., Haluskey J., Peritt D. Induction of interleukin 12 responsiveness is impaired in anergic T lymphocytes. J Exp Med. 1994 Mar 1;179(3):1065–1070. doi: 10.1084/jem.179.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecker H. C., MacDermott R. P., Mirau S., Dignass A., Podolsky D. K. Intestinal epithelial cells both express and respond to interleukin 15. Gastroenterology. 1996 Dec;111(6):1706–1713. doi: 10.1016/s0016-5085(96)70036-7. [DOI] [PubMed] [Google Scholar]
- Reinecker H. C., Podolsky D. K. Human intestinal epithelial cells express functional cytokine receptors sharing the common gamma c chain of the interleukin 2 receptor. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8353–8357. doi: 10.1073/pnas.92.18.8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S., MacDermott R. P., Raedler A., Pinnau R., Bertovich M. J., Nash G. S. Increased activation of isolated intestinal lamina propria mononuclear cells in inflammatory bowel disease. Gastroenterology. 1991 Oct;101(4):1020–1030. doi: 10.1016/0016-5085(91)90729-5. [DOI] [PubMed] [Google Scholar]
- Seder R. A., Gazzinelli R., Sher A., Paul W. E. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder R. A., Paul W. E. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- Seder R. A., Paul W. E., Davis M. M., Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992 Oct 1;176(4):1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stötter H., Custer M. C., Bolton E. S., Guedez L., Lotze M. T. IL-7 induces human lymphokine-activated killer cell activity and is regulated by IL-4. J Immunol. 1991 Jan 1;146(1):150–155. [PubMed] [Google Scholar]
- Tagaya Y., Bamford R. N., DeFilippis A. P., Waldmann T. A. IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity. 1996 Apr;4(4):329–336. doi: 10.1016/s1074-7613(00)80246-0. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Ueno Y., Yajima T., Iwao Y., Tsuchiya M., Ishikawa H., Aiso S., Hibi T., Ishii H. Interleukin 7 is produced by human intestinal epithelial cells and regulates the proliferation of intestinal mucosal lymphocytes. J Clin Invest. 1995 Jun;95(6):2945–2953. doi: 10.1172/JCI118002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Ueno Y., Yajima T., Okamoto S., Hayashi T., Yamazaki M., Iwao Y., Ishii H., Habu S., Uehira M. Interleukin 7 transgenic mice develop chronic colitis with decreased interleukin 7 protein accumulation in the colonic mucosa. J Exp Med. 1998 Feb 2;187(3):389–402. doi: 10.1084/jem.187.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West G. A., Matsuura T., Levine A. D., Klein J. S., Fiocchi C. Interleukin 4 in inflammatory bowel disease and mucosal immune reactivity. Gastroenterology. 1996 Jun;110(6):1683–1695. doi: 10.1053/gast.1996.v110.pm8964392. [DOI] [PubMed] [Google Scholar]
- Wolf S. F., Temple P. A., Kobayashi M., Young D., Dicig M., Lowe L., Dzialo R., Fitz L., Ferenz C., Hewick R. M. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991 May 1;146(9):3074–3081. [PubMed] [Google Scholar]
- Wu C. Y., Warrier R. R., Carvajal D. M., Chua A. O., Minetti L. J., Chizzonite R., Mongini P. K., Stern A. S., Gubler U., Presky D. H. Biological function and distribution of human interleukin-12 receptor beta chain. Eur J Immunol. 1996 Feb;26(2):345–350. doi: 10.1002/eji.1830260212. [DOI] [PubMed] [Google Scholar]
- Wu C., Ferrante J., Gately M. K., Magram J. Characterization of IL-12 receptor beta1 chain (IL-12Rbeta1)-deficient mice: IL-12Rbeta1 is an essential component of the functional mouse IL-12 receptor. J Immunol. 1997 Aug 15;159(4):1658–1665. [PubMed] [Google Scholar]
- Yanagida T., Kato T., Igarashi O., Inoue T., Nariuchi H. Second signal activity of IL-12 on the proliferation and IL-2R expression of T helper cell-1 clone. J Immunol. 1994 May 15;152(10):4919–4928. [PubMed] [Google Scholar]
- Zou J., Presky D. H., Wu C. Y., Gubler U. Differential associations between the cytoplasmic regions of the interleukin-12 receptor subunits beta1 and beta2 and JAK kinases. J Biol Chem. 1997 Feb 28;272(9):6073–6077. doi: 10.1074/jbc.272.9.6073. [DOI] [PubMed] [Google Scholar]