Abstract
Background—The matrix metalloproteinases (MMPs) and tissue inhibitors of matrix metalloproteinases (TIMPs) are strongly implicated in tumour invasion and metastasis. Aims—To investigate the presence of individual MMPs and TIMPs in gastric cancer. Methods—The presence of MMP-1, MMP-2, MMP-3, MMP-9, TIMP-1, and TIMP-2 was identified in a group of gastric cancers (n=74) by immunohistochemistry using monoclonal antibodies. These antibodies were effective on formalin fixed, paraffin wax embedded sections. Results—A large proportion (94%) of gastric cancers contained MMP-2; MMP-1 and MMP-9 were also detected in 73% and 70% of tumours respectively. MMP-3 was only present in 27% of tumours. MMP-1 and MMP-9 were found predominantly in intestinal type tumours. TIMP-1 and TIMP-2 were identified in 41% and 57% of tumours respectively. Immunoreactivity for individual MMPs or TIMPs was not identified in normal stomach. Conclusions—This study shows the presence of matrix metalloproteinases, particularly MMP-2, and TIMPs in stomach cancer. Antibodies which are effective in formalin fixed, paraffin wax embedded sections are useful for the identification of MMPs and TIMPs in diagnostic specimens.
Keywords: immunohistochemistry; matrix metalloproteinase; neoplasm; stomach
Full Text
The Full Text of this article is available as a PDF (239.6 KB).
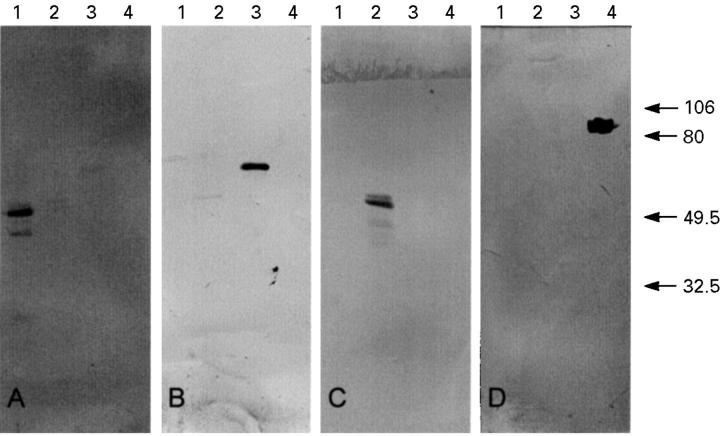
Figure 1 .
Immunoblot showing the specificity of the MMP-1, MMP-2, MMP-3, and MMP-9 antibodies. The membrane was immunostained with MMP-1 (A), MMP-2 (B), MMP-3 (C), and MMP-9 antibody (D). Lane 1, MMP-1; lane 2, MMP-3; lane 3, MMP-2; lane 4, MMP-9. Molecular weight markers are shown on the right in kDa.
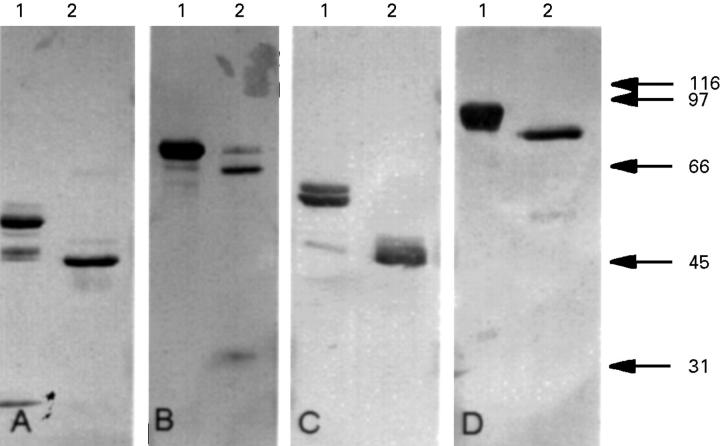
Figure 2 .
Immunoblots showing the reaction of the MMP-1, MMP-2, MMP-3, and MMP-9 monoclonal antibodies with the proenzyme and activated forms of the corresponding MMPs. The membrane was immunostained with MMP-1 (A), MMP-2 (B), MMP-3 (C), and MMP-9 antibody (D). Lane 1 contained the proenzyme and lane 2 the activated form of the MMP corresponding to the monoclonal antibody used for the immunostaining. Molecular weight markers are shown on the right in kDa.
Figure 3 .
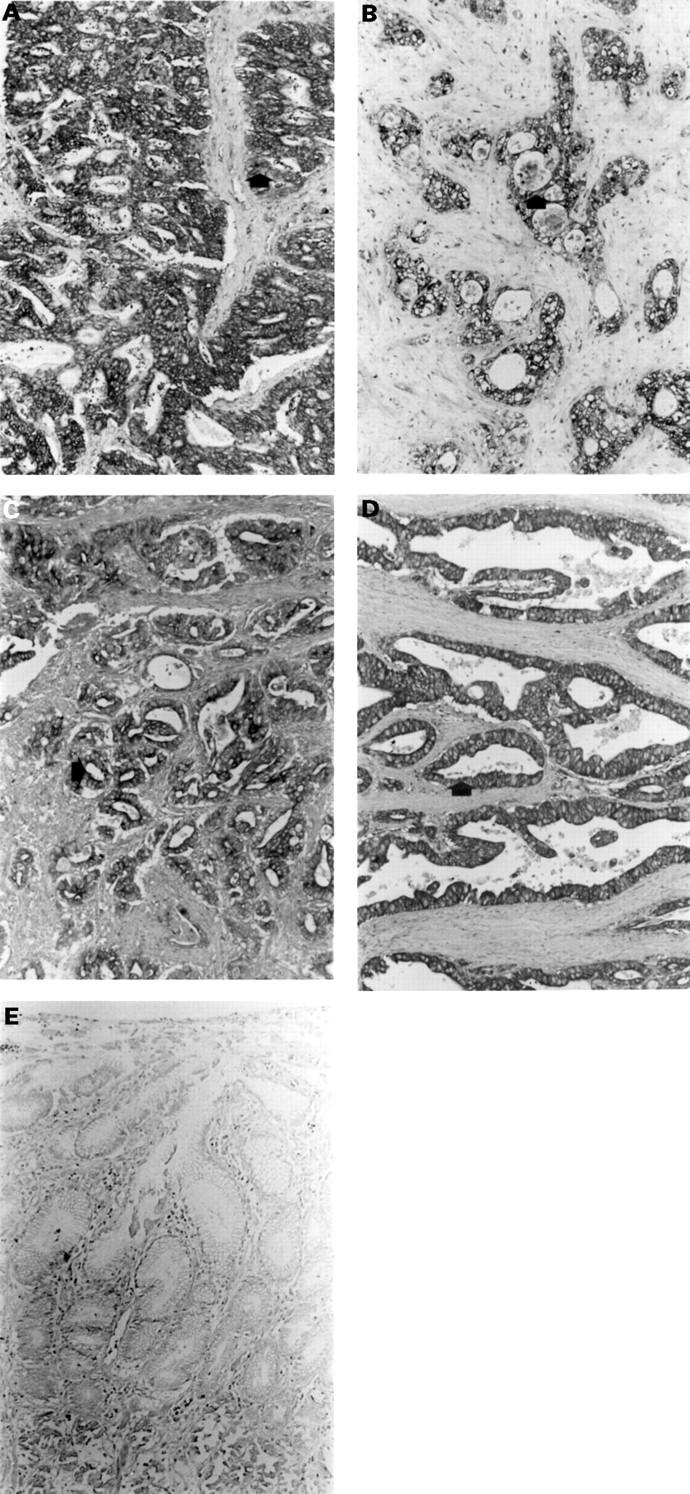
Immunohistochemical localisation of: (A) MMP-1; (B) MMP-2; (C) MMP-3; (D) MMP-9 in gastric cancers of intestinal type; and (E) MMP-1 in normal gastric epithelium (similar results were obtained with antibodies to the other MMPs). Strong immunoreactivity for each MMP is present in tumour cells (arrows) and is not present in normal gastric epithelium (original magnification ×120).
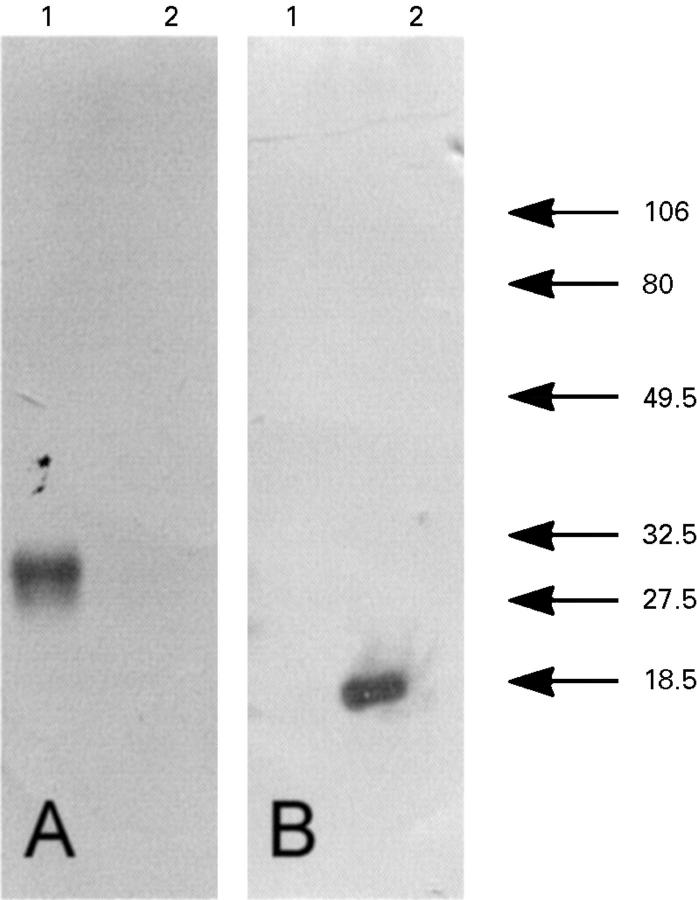
Figure 4 .
Immunoblot showing the specificity of the TIMP-1 and TIMP-2 antibodies. Section A was immunostained with TIMP-1 antibody and section B with TIMP-2 antibody. Lane 1, TIMP-1; lane 2, TIMP-2. Molecular weight markers are shown on the right in kDa.
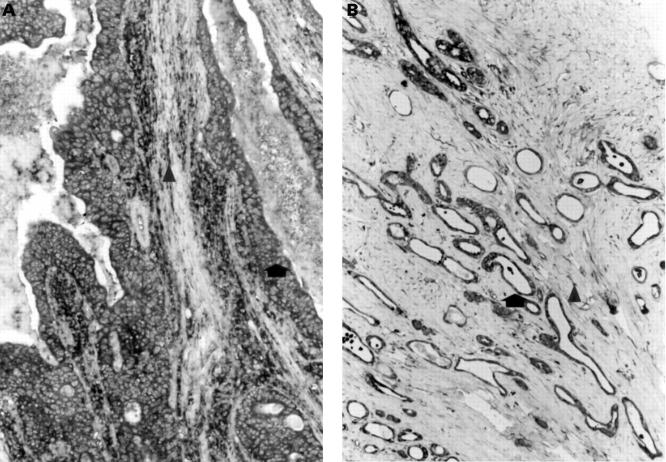
Figure 5 .
Immunohistochemical localisation of (A) TIMP-1 and (B) TIMP-2 in gastric cancers of intestinal type. TIMP-1 and TIMP-2 immunoreactivity is present in tumour cells (arrows). Immunostaining for TIMP-1 is also present in macrophages (arrowhead) and immunostaining for TIMP-2 is also present in fibroblasts (arrowhead) (original magnification ×120).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini A., Melchiori A., Santi L., Liotta L. A., Brown P. D., Stetler-Stevenson W. G. Tumor cell invasion inhibited by TIMP-2. J Natl Cancer Inst. 1991 Jun 5;83(11):775–779. doi: 10.1093/jnci/83.11.775. [DOI] [PubMed] [Google Scholar]
- Boag A. H., Young I. D. Increased expression of the 72-kd type IV collagenase in prostatic adenocarcinoma. Demonstration by immunohistochemistry and in situ hybridization. Am J Pathol. 1994 Mar;144(3):585–591. [PMC free article] [PubMed] [Google Scholar]
- Brown P. D., Giavazzi R. Matrix metalloproteinase inhibition: a review of anti-tumour activity. Ann Oncol. 1995 Dec;6(10):967–974. doi: 10.1093/oxfordjournals.annonc.a059091. [DOI] [PubMed] [Google Scholar]
- Cawston T. E. Metalloproteinase inhibitors and the prevention of connective tissue breakdown. Pharmacol Ther. 1996;70(3):163–182. doi: 10.1016/0163-7258(96)00015-0. [DOI] [PubMed] [Google Scholar]
- David L., Nesland J. M., Holm R., Sobrinho-Simões M. Expression of laminin, collagen IV, fibronectin, and type IV collagenase in gastric carcinoma. An immunohistochemical study of 87 patients. Cancer. 1994 Feb 1;73(3):518–527. doi: 10.1002/1097-0142(19940201)73:3<518::aid-cncr2820730305>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Davies B., Waxman J., Wasan H., Abel P., Williams G., Krausz T., Neal D., Thomas D., Hanby A., Balkwill F. Levels of matrix metalloproteases in bladder cancer correlate with tumor grade and invasion. Cancer Res. 1993 Nov 15;53(22):5365–5369. [PubMed] [Google Scholar]
- Denhardt D. T., Feng B., Edwards D. R., Cocuzzi E. T., Malyankar U. M. Tissue inhibitor of metalloproteinases (TIMP, aka EPA): structure, control of expression and biological functions. Pharmacol Ther. 1993 Sep;59(3):329–341. doi: 10.1016/0163-7258(93)90074-n. [DOI] [PubMed] [Google Scholar]
- Douglas D. A., Shi Y. E., Sang Q. A. Computational sequence analysis of the tissue inhibitor of metalloproteinase family. J Protein Chem. 1997 May;16(4):237–255. doi: 10.1023/a:1026348808069. [DOI] [PubMed] [Google Scholar]
- Duncan M. E., McAleese S. M., Booth N. A., Melvin W. T., Fothergill J. E. A simple enzyme-linked immunosorbent assay (ELISA) for the neuron-specific gamma isozyme of human enolase (NSE) using monoclonal antibodies raised against synthetic peptides corresponding to isozyme sequence differences. J Immunol Methods. 1992 Jul 6;151(1-2):227–236. doi: 10.1016/0022-1759(92)90121-9. [DOI] [PubMed] [Google Scholar]
- Gomis-Rüth F. X., Maskos K., Betz M., Bergner A., Huber R., Suzuki K., Yoshida N., Nagase H., Brew K., Bourenkov G. P. Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 by TIMP-1. Nature. 1997 Sep 4;389(6646):77–81. doi: 10.1038/37995. [DOI] [PubMed] [Google Scholar]
- Greene J., Wang M., Liu Y. E., Raymond L. A., Rosen C., Shi Y. E. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem. 1996 Nov 29;271(48):30375–30380. doi: 10.1074/jbc.271.48.30375. [DOI] [PubMed] [Google Scholar]
- Hart I. R., Saini A. Biology of tumour metastasis. Lancet. 1992 Jun 13;339(8807):1453–1457. doi: 10.1016/0140-6736(92)92039-i. [DOI] [PubMed] [Google Scholar]
- Hiraga A., Kemp B. E., Cohen P. Further studies on the structure of the glycogen-bound form of protein phosphatase-1 from rabbit skeletal muscle. Eur J Biochem. 1987 Mar 2;163(2):253–258. doi: 10.1111/j.1432-1033.1987.tb10795.x. [DOI] [PubMed] [Google Scholar]
- Hua J., Muschel R. J. Inhibition of matrix metalloproteinase 9 expression by a ribozyme blocks metastasis in a rat sarcoma model system. Cancer Res. 1996 Nov 15;56(22):5279–5284. [PubMed] [Google Scholar]
- Höyhtyä M., Fridman R., Komarek D., Porter-Jordan K., Stetler-Stevenson W. G., Liotta L. A., Liang C. M. Immunohistochemical localization of matrix metalloproteinase 2 and its specific inhibitor TIMP-2 in neoplastic tissues with monoclonal antibodies. Int J Cancer. 1994 Feb 15;56(4):500–505. doi: 10.1002/ijc.2910560408. [DOI] [PubMed] [Google Scholar]
- Kawano N., Osawa H., Ito T., Nagashima Y., Hirahara F., Inayama Y., Nakatani Y., Kimura S., Kitajima H., Koshikawa N. Expression of gelatinase A, tissue inhibitor of metalloproteinases-2, matrilysin, and trypsin(ogen) in lung neoplasms: an immunohistochemical study. Hum Pathol. 1997 May;28(5):613–622. doi: 10.1016/s0046-8177(97)90085-x. [DOI] [PubMed] [Google Scholar]
- Kohn E. C., Liotta L. A. Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res. 1995 May 1;55(9):1856–1862. [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Muller D., Wolf C., Abecassis J., Millon R., Engelmann A., Bronner G., Rouyer N., Rio M. C., Eber M., Methlin G. Increased stromelysin 3 gene expression is associated with increased local invasiveness in head and neck squamous cell carcinomas. Cancer Res. 1993 Jan 1;53(1):165–169. [PubMed] [Google Scholar]
- Murphy G., Docherty A. J., Hembry R. M., Reynolds J. J. Metalloproteinases and tissue damage. Br J Rheumatol. 1991;30 (Suppl 1):25–31. [PubMed] [Google Scholar]
- Murphy G., Docherty A. J. The matrix metalloproteinases and their inhibitors. Am J Respir Cell Mol Biol. 1992 Aug;7(2):120–125. doi: 10.1165/ajrcmb/7.2.120. [DOI] [PubMed] [Google Scholar]
- Murray G. I., Duncan M. E., Melvin W. T., Fothergill J. E. Immunohistochemistry of neurone specific enolase with gamma subunit specific anti-peptide monoclonal antibodies. J Clin Pathol. 1993 Nov;46(11):993–996. doi: 10.1136/jcp.46.11.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray G. I., Duncan M. E., O'Neil P., McKay J. A., Melvin W. T., Fothergill J. E. Matrix metalloproteinase-1 is associated with poor prognosis in oesophageal cancer. J Pathol. 1998 Jul;185(3):256–261. doi: 10.1002/(SICI)1096-9896(199807)185:3<256::AID-PATH115>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Murray G. I., Duncan M. E., O'Neil P., Melvin W. T., Fothergill J. E. Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat Med. 1996 Apr;2(4):461–462. doi: 10.1038/nm0496–461. [DOI] [PubMed] [Google Scholar]
- Nomura H., Fujimoto N., Seiki M., Mai M., Okada Y. Enhanced production of matrix metalloproteinases and activation of matrix metalloproteinase 2 (gelatinase A) in human gastric carcinomas. Int J Cancer. 1996 Feb 20;69(1):9–16. doi: 10.1002/(SICI)1097-0215(19960220)69:1<9::AID-IJC3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Sato H., Seiki M. Membrane-type matrix metalloproteinases (MT-MMPs) in tumor metastasis. J Biochem. 1996 Feb;119(2):209–215. doi: 10.1093/oxfordjournals.jbchem.a021223. [DOI] [PubMed] [Google Scholar]
- Sato H., Takino T., Okada Y., Cao J., Shinagawa A., Yamamoto E., Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994 Jul 7;370(6484):61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- Schwartz G. K., Wang H., Lampen N., Altorki N., Kelsen D., Albino A. P. Defining the invasive phenotype of proximal gastric cancer cells. Cancer. 1994 Jan 1;73(1):22–27. doi: 10.1002/1097-0142(19940101)73:1<22::aid-cncr2820730106>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Sier C. F., Kubben F. J., Ganesh S., Heerding M. M., Griffioen G., Hanemaaijer R., van Krieken J. H., Lamers C. B., Verspaget H. W. Tissue levels of matrix metalloproteinases MMP-2 and MMP-9 are related to the overall survival of patients with gastric carcinoma. Br J Cancer. 1996 Aug;74(3):413–417. doi: 10.1038/bjc.1996.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sledge G. W., Jr, Qulali M., Goulet R., Bone E. A., Fife R. Effect of matrix metalloproteinase inhibitor batimastat on breast cancer regrowth and metastasis in athymic mice. J Natl Cancer Inst. 1995 Oct 18;87(20):1546–1550. doi: 10.1093/jnci/87.20.1546. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Liotta L. A., Kleiner D. E., Jr Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. FASEB J. 1993 Dec;7(15):1434–1441. doi: 10.1096/fasebj.7.15.8262328. [DOI] [PubMed] [Google Scholar]
- Tryggvason K., Höyhtyä M., Pyke C. Type IV collagenases in invasive tumors. Breast Cancer Res Treat. 1993;24(3):209–218. doi: 10.1007/BF01833261. [DOI] [PubMed] [Google Scholar]
- Urbanski S. J., Edwards D. R., Hershfield N., Huchcroft S. A., Shaffer E., Sutherland L., Kossakowska A. E. Expression pattern of metalloproteinases and their inhibitors changes with the progression of human sporadic colorectal neoplasia. Diagn Mol Pathol. 1993 Jun;2(2):81–89. [PubMed] [Google Scholar]
- Uría J. A., Ferrando A. A., Velasco G., Freije J. M., López-Otín C. Structure and expression in breast tumors of human TIMP-3, a new member of the metalloproteinase inhibitor family. Cancer Res. 1994 Apr 15;54(8):2091–2094. [PubMed] [Google Scholar]
- Watanabe M., Takahashi Y., Ohta T., Mai M., Sasaki T., Seiki M. Inhibition of metastasis in human gastric cancer cells transfected with tissue inhibitor of metalloproteinase 1 gene in nude mice. Cancer. 1996 Apr 15;77(8 Suppl):1676–1680. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1676::AID-CNCR38>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Watson S. A., Morris T. M., Robinson G., Crimmin M. J., Brown P. D., Hardcastle J. D. Inhibition of organ invasion by the matrix metalloproteinase inhibitor batimastat (BB-94) in two human colon carcinoma metastasis models. Cancer Res. 1995 Aug 15;55(16):3629–3633. [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]