Abstract
Background—Although leptin, an adipocyte derived hormone which regulates food intake and energy balance, is released after injections of tumour necrosis factor (TNF) and interleukin 1,plasma concentrations have not been characterised in chronic inflammation. Leptin may contribute to the anorexia and body weight loss associated particularly with the acute stages of inflammatory bowel disease. Aims—To investigate plasma leptin concentrations during the time course of intestinal inflammation in different animal models. Methods—Plasma leptin was measured at different time points in rats with trinitrobenzene sulphonic acid (TNBS) induced colitis, indomethacin induced ileitis, or endotoxic shock caused by lipopolysaccharide (LPS). Systemic TNF-α was also measured during acute inflammation. Results—Plasma leptin concentrations increased fourfold eight hours after induction of TNBS colitis (p<0.0001) and twofold after administration of ethanol alone (p<0.02). Plasma leptin responses throughout the first post-treatment day were correlated with myeloperoxidase activity and gross damage scores. Similar leptin overexpression was observed in indomethacin induced ileitis and in rats with endotoxic shock. Plasma concentrations were lower in TNBS treated rats than in controls on day 5 before reaching a similar concentration on day 14. Anorexia and body weight loss were observed during the first four days post-TNBS. A significant increase in systemic TNF-α was only detected in LPS treated rats. Conclusion—Elevated plasma leptin concentrations, correlated with the degree of inflammation and associated with anorexia, were induced in rats during the early stages of experimental intestinal inflammation but proved transient; this might account for discrepancies in recent results concerning concentrations in patients with inflammatory bowel diseases.
Keywords: leptin; inflammatory bowel disease; experimental rat intestinal inflammation; tumour necrosis factor; endotoxic shock; anorexia
Full Text
The Full Text of this article is available as a PDF (159.3 KB).
Figure 1 .
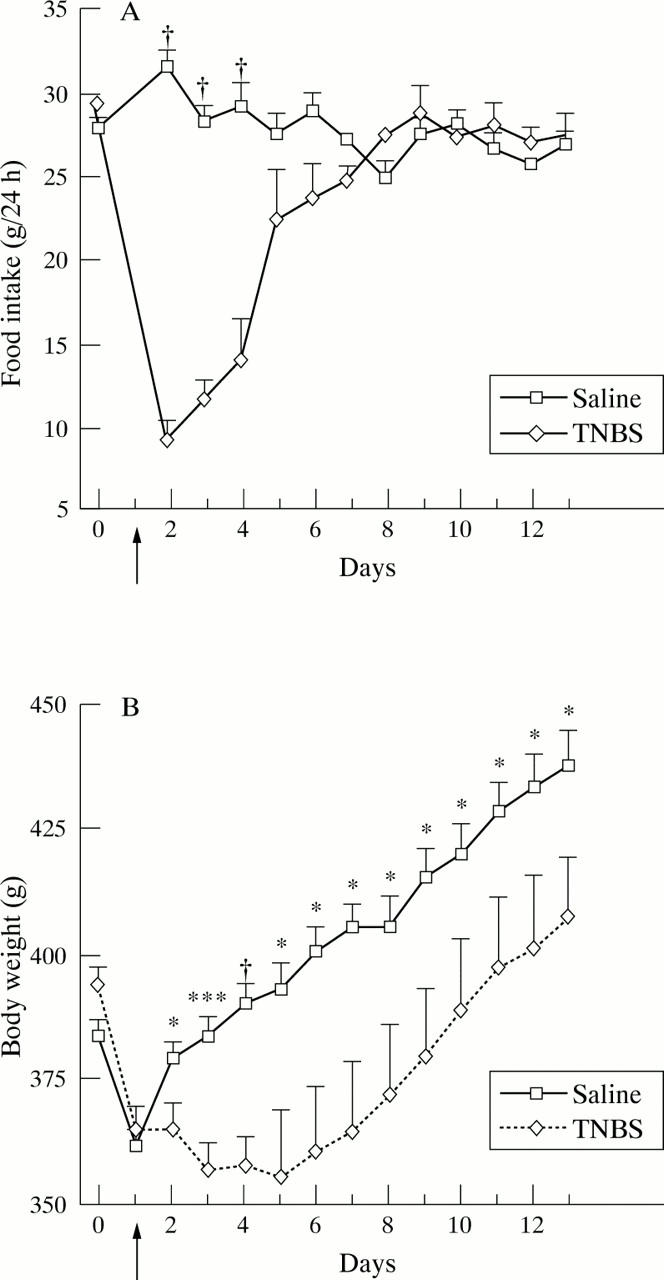
Daily (24 h) food intake (A) and body weight (B) for 13 days after intracolonic administration (arrow) of TNBS or saline in rats. Day 0 represents pretreatment baseline. Values are means (SEM). Significantly different between saline and TNBS, *p<0.05, ***p<0.001, †p<0.0001.
Figure 2 .
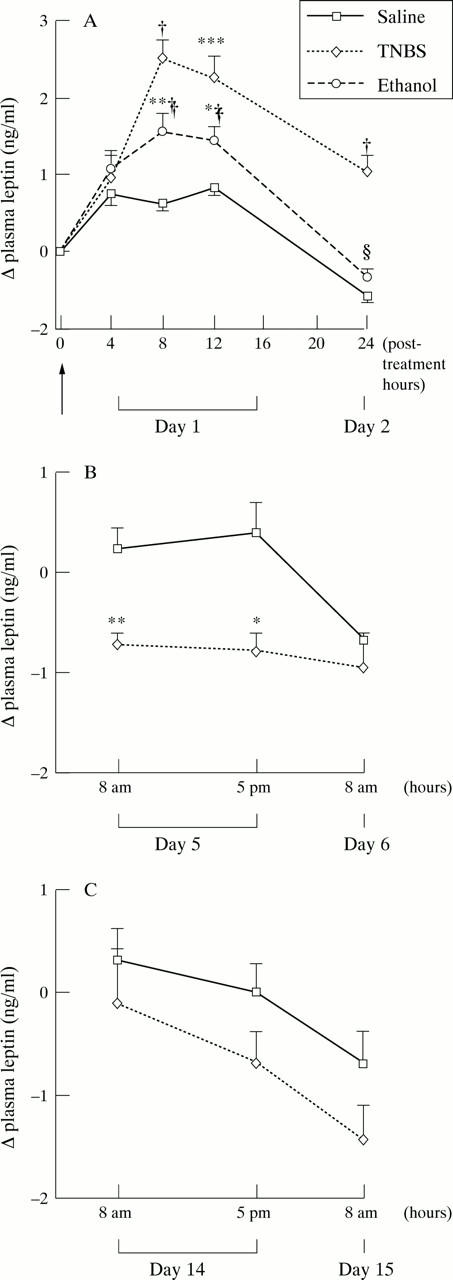
Changes in plasma leptin concentrations after intracolonic administration (arrow) of TNBS, ethanol, or saline in rats on days 1 and 2 (A), days 5 and 6 (B), and days 14 and 15 (C). Values are means (SEM). Significantly different from saline: *p<0.05, **p<0.01, ***p<0.001, †p<0.0001. Significantly different from TNBS, ‡p<0.05, §p<0.001.
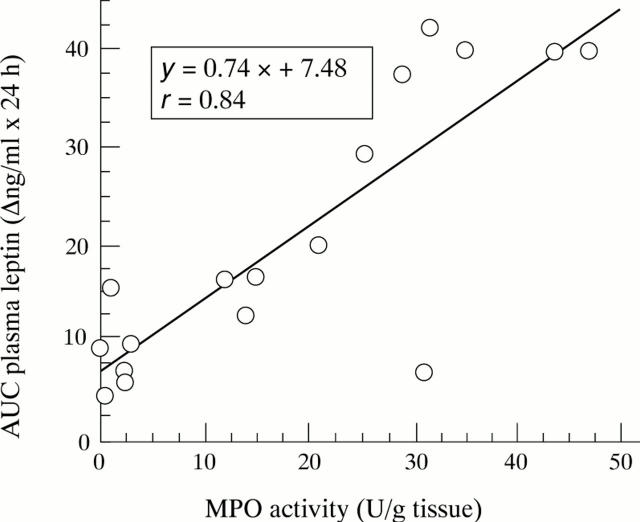
Figure 3 .
Correlation of areas under the curve (AUC) of changes in plasma leptin concentrations throughout the first day post-TNBS, post-ethanol, and post-saline with MPO activity measured at 24 hours post-treatment. Each point represents the value of one rat (n=17), p<0.0001.
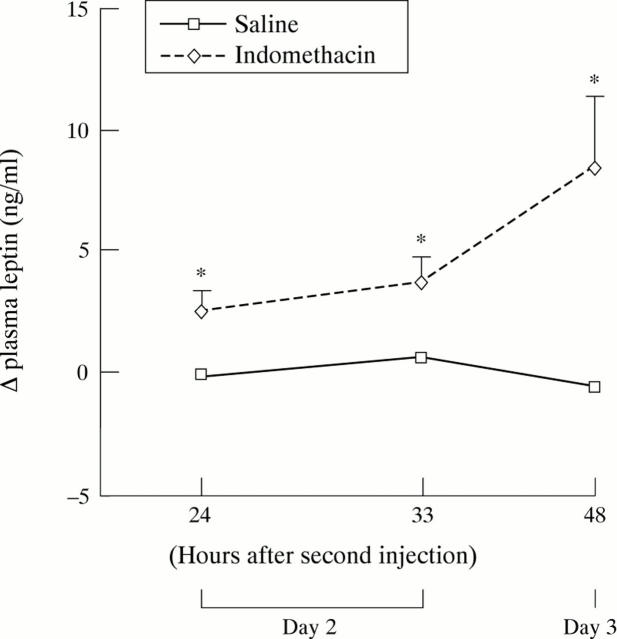
Figure 4 .
Changes in plasma leptin concentrations on days 2 and 3 in the indomethacin induced ileitis model. All rats were subcutaneously injected with indomethacin (n=7) or saline (n=6) daily at 24 hour intervals, the second injection representing day 1. Values are means (SEM). *Significantly different from saline, p<0.05.
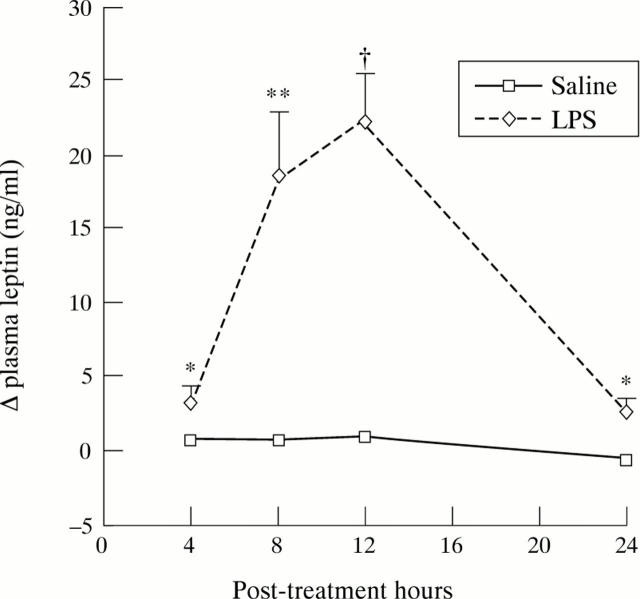
Figure 5 .
Changes in plasma leptin concentrations on the first day after intraperitoneal administration of lipopolysaccharide (n=6) or saline (n=6) in rats. Values are means (SEM). Significantly different from saline, *p<0.05, **p<0.01, †p<0.001.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ameho C. K., Adjei A. A., Harrison E. K., Takeshita K., Morioka T., Arakaki Y., Ito E., Suzuki I., Kulkarni A. D., Kawajiri A. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut. 1997 Oct;41(4):487–493. doi: 10.1136/gut.41.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campfield L. A., Smith F. J., Guisez Y., Devos R., Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995 Jul 28;269(5223):546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- Chehab F. F., Mounzih K., Lu R., Lim M. E. Early onset of reproductive function in normal female mice treated with leptin. Science. 1997 Jan 3;275(5296):88–90. doi: 10.1126/science.275.5296.88. [DOI] [PubMed] [Google Scholar]
- Cioffi J. A., Shafer A. W., Zupancic T. J., Smith-Gbur J., Mikhail A., Platika D., Snodgrass H. R. Novel B219/OB receptor isoforms: possible role of leptin in hematopoiesis and reproduction. Nat Med. 1996 May;2(5):585–589. doi: 10.1038/nm0596-585. [DOI] [PubMed] [Google Scholar]
- Considine R. V., Sinha M. K., Heiman M. L., Kriauciunas A., Stephens T. W., Nyce M. R., Ohannesian J. P., Marco C. C., McKee L. J., Bauer T. L. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996 Feb 1;334(5):292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- De Vos P., Saladin R., Auwerx J., Staels B. Induction of ob gene expression by corticosteroids is accompanied by body weight loss and reduced food intake. J Biol Chem. 1995 Jul 7;270(27):15958–15961. doi: 10.1074/jbc.270.27.15958. [DOI] [PubMed] [Google Scholar]
- Dieleman L. A., Peña A. S., Meuwissen S. G., van Rees E. P. Role of animal models for the pathogenesis and treatment of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1997;223:99–104. [PubMed] [Google Scholar]
- Gainsford T., Willson T. A., Metcalf D., Handman E., McFarlane C., Ng A., Nicola N. A., Alexander W. S., Hilton D. J. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14564–14568. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenwald J. E., Fong Y. M., Fahey T. J., 3rd, Calvano S. E., Chizzonite R., Kilian P. L., Lowry S. F., Moldawer L. L. Interleukin 1 receptor blockade attenuates the host inflammatory response. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4966–4970. doi: 10.1073/pnas.87.13.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghilardi N., Ziegler S., Wiestner A., Stoffel R., Heim M. H., Skoda R. C. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci U S A. 1996 Jun 25;93(13):6231–6235. doi: 10.1073/pnas.93.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granowitz E. V. Transforming growth factor-beta enhances and pro-inflammatory cytokines inhibit ob gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1997 Nov 17;240(2):382–385. doi: 10.1006/bbrc.1997.7663. [DOI] [PubMed] [Google Scholar]
- Grunfeld C., Zhao C., Fuller J., Pollack A., Moser A., Friedman J., Feingold K. R. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest. 1996 May 1;97(9):2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaas J. L., Gajiwala K. S., Maffei M., Cohen S. L., Chait B. T., Rabinowitz D., Lallone R. L., Burley S. K., Friedman J. M. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995 Jul 28;269(5223):543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Janik J. E., Curti B. D., Considine R. V., Rager H. C., Powers G. C., Alvord W. G., Smith J. W., 2nd, Gause B. L., Kopp W. C. Interleukin 1 alpha increases serum leptin concentrations in humans. J Clin Endocrinol Metab. 1997 Sep;82(9):3084–3086. doi: 10.1210/jcem.82.9.4214. [DOI] [PubMed] [Google Scholar]
- Kirchgessner T. G., Uysal K. T., Wiesbrock S. M., Marino M. W., Hotamisligil G. S. Tumor necrosis factor-alpha contributes to obesity-related hyperleptinemia by regulating leptin release from adipocytes. J Clin Invest. 1997 Dec 1;100(11):2777–2782. doi: 10.1172/JCI119824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawisz J. E., Sharon P., Stenson W. F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984 Dec;87(6):1344–1350. [PubMed] [Google Scholar]
- Löllmann B., Grüninger S., Stricker-Krongrad A., Chiesi M. Detection and quantification of the leptin receptor splice variants Ob-Ra, b, and, e in different mouse tissues. Biochem Biophys Res Commun. 1997 Sep 18;238(2):648–652. doi: 10.1006/bbrc.1997.7205. [DOI] [PubMed] [Google Scholar]
- Madej T., Boguski M. S., Bryant S. H. Threading analysis suggests that the obese gene product may be a helical cytokine. FEBS Lett. 1995 Oct 2;373(1):13–18. doi: 10.1016/0014-5793(95)00977-h. [DOI] [PubMed] [Google Scholar]
- Mantzoros C. S., Moschos S., Avramopoulos I., Kaklamani V., Liolios A., Doulgerakis D. E., Griveas I., Katsilambros N., Flier J. S. Leptin concentrations in relation to body mass index and the tumor necrosis factor-alpha system in humans. J Clin Endocrinol Metab. 1997 Oct;82(10):3408–3413. doi: 10.1210/jcem.82.10.4323. [DOI] [PubMed] [Google Scholar]
- Martinolle J. P., Garcia-Villar R., Fioramonti J., Bueno L. Altered contractility of circular and longitudinal muscle in TNBS-inflamed guinea pig ileum. Am J Physiol. 1997 May;272(5 Pt 1):G1258–G1267. doi: 10.1152/ajpgi.1997.272.5.G1258. [DOI] [PubMed] [Google Scholar]
- McHugh K. J., Collins S. M., Weingarten H. P. Central interleukin-1 receptors contribute to suppression of feeding after acute colitis in the rat. Am J Physiol. 1994 May;266(5 Pt 2):R1659–R1663. doi: 10.1152/ajpregu.1994.266.5.R1659. [DOI] [PubMed] [Google Scholar]
- Mingrone G., Greco A. V., Benedetti G., Capristo E., Semeraro R., Zoli G., Gasbarrini G. Increased resting lipid oxidation in Crohn's disease. Dig Dis Sci. 1996 Jan;41(1):72–76. doi: 10.1007/BF02208586. [DOI] [PubMed] [Google Scholar]
- Morris G. P., Beck P. L., Herridge M. S., Depew W. T., Szewczuk M. R., Wallace J. L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989 Mar;96(3):795–803. [PubMed] [Google Scholar]
- Neilly P. J., Gardiner K. R., Kirk S. J., Jennings G., Anderson N. H., Elia M., Rowlands B. J. Endotoxaemia and cytokine production in experimental colitis. Br J Surg. 1995 Nov;82(11):1479–1482. doi: 10.1002/bjs.1800821110. [DOI] [PubMed] [Google Scholar]
- O'Reilly B., Vander A. J., Kluger M. J. Effects of chronic infusion of lipopolysaccharide on food intake and body temperature of the rat. Physiol Behav. 1988;42(3):287–291. doi: 10.1016/0031-9384(88)90084-4. [DOI] [PubMed] [Google Scholar]
- Papaspyrou-Rao S., Schneider S. H., Petersen R. N., Fried S. K. Dexamethasone increases leptin expression in humans in vivo. J Clin Endocrinol Metab. 1997 May;82(5):1635–1637. doi: 10.1210/jcem.82.5.3928. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D., Simon P. L., Schwartz L. W., Griswold D. E., Fondacaro J. D., Wasserman M. A. Inflammatory mediators of experimental colitis in rats. Gastroenterology. 1989 Aug;97(2):326–337. doi: 10.1016/0016-5085(89)90068-1. [DOI] [PubMed] [Google Scholar]
- Rigaud D., Angel L. A., Cerf M., Carduner M. J., Melchior J. C., Sautier C., René E., Apfelbaum M., Mignon M. Mechanisms of decreased food intake during weight loss in adult Crohn's disease patients without obvious malabsorption. Am J Clin Nutr. 1994 Nov;60(5):775–781. doi: 10.1093/ajcn/60.5.775. [DOI] [PubMed] [Google Scholar]
- Sarraf P., Frederich R. C., Turner E. M., Ma G., Jaskowiak N. T., Rivet D. J., 3rd, Flier J. S., Lowell B. B., Fraker D. L., Alexander H. R. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997 Jan 6;185(1):171–175. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk D. B., Payne-James J. Inflammatory bowel disease: nutritional implications and treatment. Proc Nutr Soc. 1989 Sep;48(3):355–361. doi: 10.1079/pns19890051. [DOI] [PubMed] [Google Scholar]
- Tartaglia L. A., Dembski M., Weng X., Deng N., Culpepper J., Devos R., Richards G. J., Campfield L. A., Clark F. T., Deeds J. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995 Dec 29;83(7):1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Tateishi H., Mitsuyama K., Toyonaga A., Tomoyose M., Tanikawa K. Role of cytokines in experimental colitis: relation to intestinal permeability. Digestion. 1997;58(3):271–281. doi: 10.1159/000201454. [DOI] [PubMed] [Google Scholar]
- Uchida A., Yamada T., Hayakawa T., Hoshino M. Taurochenodeoxycholic acid ameliorates and ursodeoxycholic acid exacerbates small intestinal inflammation. Am J Physiol. 1997 May;272(5 Pt 1):G1249–G1257. doi: 10.1152/ajpgi.1997.272.5.G1249. [DOI] [PubMed] [Google Scholar]
- Weigle D. S., Duell P. B., Connor W. E., Steiner R. A., Soules M. R., Kuijper J. L. Effect of fasting, refeeding, and dietary fat restriction on plasma leptin levels. J Clin Endocrinol Metab. 1997 Feb;82(2):561–565. doi: 10.1210/jcem.82.2.3757. [DOI] [PubMed] [Google Scholar]
- Yamada T., Deitch E., Specian R. D., Perry M. A., Sartor R. B., Grisham M. B. Mechanisms of acute and chronic intestinal inflammation induced by indomethacin. Inflammation. 1993 Dec;17(6):641–662. doi: 10.1007/BF00920471. [DOI] [PubMed] [Google Scholar]
- Zumbach M. S., Boehme M. W., Wahl P., Stremmel W., Ziegler R., Nawroth P. P. Tumor necrosis factor increases serum leptin levels in humans. J Clin Endocrinol Metab. 1997 Dec;82(12):4080–4082. doi: 10.1210/jcem.82.12.4408. [DOI] [PubMed] [Google Scholar]