Abstract
Background—Death in the early stages of severe acute pancreatitis is frequently the result of multiple organ dysfunction, but its mechanism is not clear. Aims—To investigate the state of nuclear factor-κB (NF-κB) in macrophages of rats with lethal pancreatitis, and to assess the effectiveness of pyrrolidine dithiocarbamate, an inhibitor of NF-κB, on the pathology and mortality. Methods—Taurocholate pancreatitis was produced in rats, and the severity of the disease, the mortality, and activation of NF-κB in peritoneal and alveolar macrophages were compared in rats receiving pyrrolidine dithiocarbamate (PDTC) treatment and those that were not. Results—Taurocholate pancreatitis produced massive necrosis, haemorrhage, and severe leucocyte infiltration in the pancreas as well as alveolar septal thickening in the lung. NF-κB was activated in peritoneal and alveolar macrophages six hours after pancreatitis induction. Pretreatment with PDTC dose-dependently attenuated the NF-κB activation and improved the survival of the rats, although it did not affect the early increase in serum amylase and histological findings. Conclusions—Early blockage of NF-κB activation may be effective in reducing fatal outcome in severe acute pancreatitis.
Keywords: pancreatitis; multiple organ dysfunction; nuclear factor-κB; pyrrolidine dithiocarbamate; macrophages; rat
Full Text
The Full Text of this article is available as a PDF (223.3 KB).
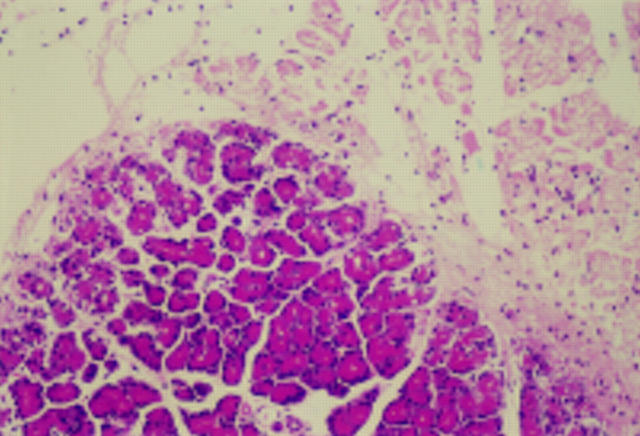
Figure 1 .
Histological findings for the pancreas (A), lung (B), and liver (C) 24 hours after the induction of taurocholate (TCA) pancreatitis. Original magnification × 40.
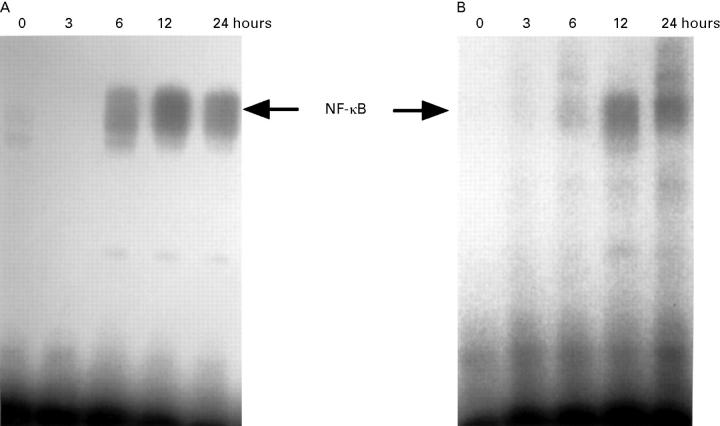
Figure 2 .
Electrophoretic mobility shift assay of NF-κB using the 32P labelled probe. Peritoneal macrophages (A) and alveolar macrophages (B) were collected from rats with taurocholate pancreatitis at the indicated time after the induction of pancreatitis.
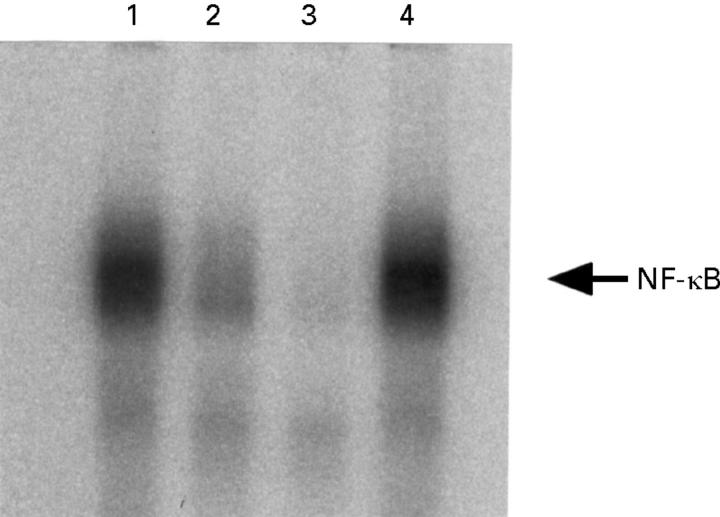
Figure 3 .
Competition assays of the activated NF-κB. Peritoneal macrophages were collected 12 hours after the induction of taurocholate pancreatitis. Nuclear extracts were preincubated with no reagents (lane 1), a 10-fold (lane 2) or a 100-fold (lane 3) molar excess of unlabelled κB oligonucleotide, or a 100-fold molar excess of mutant κB oligonucleotide (lane 4). In the competition assay, an excess of the oligonucleotide competitor was preincubated with the nuclear extract for 60 minutes at room temperature.
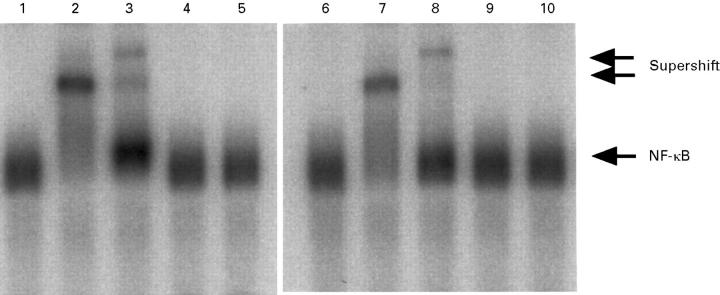
Figure 4 .
Supershift assays of activated NF-κB. Peritoneal macrophages (lanes 1-5) and alveolar macrophages (lanes 6-10) were collected 12 hours after the induction of taurocholate pancreatitis. Nuclear extracts were preincubated with no reagents (lanes 1 and 6), or with 2 µg each of the rabbit polyclonal antibody against p50 (lanes 2 and 7), p65 (lanes 3 and 8), c-Rel (lanes 4 and 9), or p52 (lanes 5 and 10). In the supershift assays, incubation with the respective antibody was carried out at 4°C for 60 minutes.
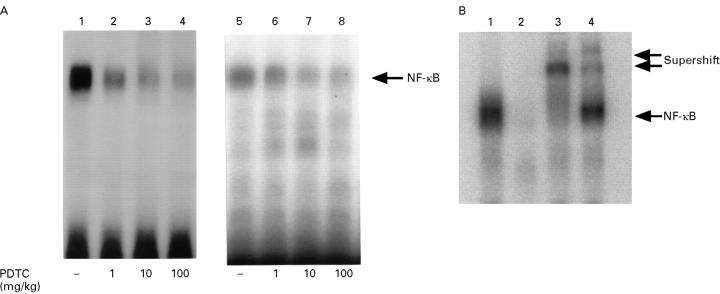
Figure 5 .
(A) Effects of pyrrolidine dithiocarbamate (PDTC) on NF-κB activation. Peritoneal macrophages (lanes 1-4) and alveolar macrophages (lanes 5-8) were obtained 12 hours after the induction of taurocholate pancreatitis. Lanes 2-4 and 6-8 show NF-κB activation in the respective type of macrophages obtained from rats pretreated with the indicated doses of PDTC (1, 10, and 100 mg/kg) one hour before the induction of pancreatitis, whereas lanes 1 and 5 show NF-κB activation in the respective type of macrophages obtained from rats not pretreated with PDTC. (B) Specificity studies of activated NF-κB in the peritoneal macrophages of pancreatitis rats pretreated with 1 mg/kg PDTC. Nuclear extracts from macrophages were preincubated with no reagents (lane 1), with a 100-fold molar excess of unlabelled κB oligonucleotide (lane 2), with 2 µg/ml of the rabbit polyclonal antibody against either p50 (lane 3) or p65 (lane 4). Then the mixtures were subjected to electrophoretic mobility shift assay.
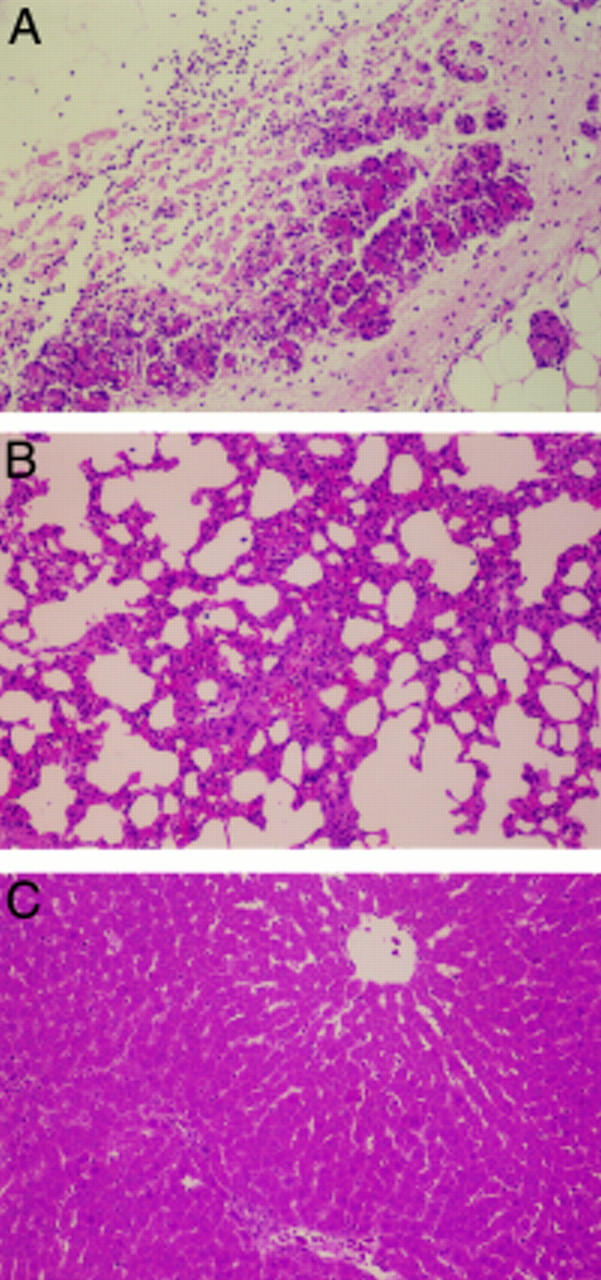
Figure 6 .
Effects of pyrrolidine dithiocarbamate (PDTC) on the histological findings in the pancreas. PDTC at a dose of 10 mg/kg was given intraperitoneally one hour before the induction of pancreatitis and the tissues were obtained 24 hours after the induction of pancreatitis. Original magnification × 40.
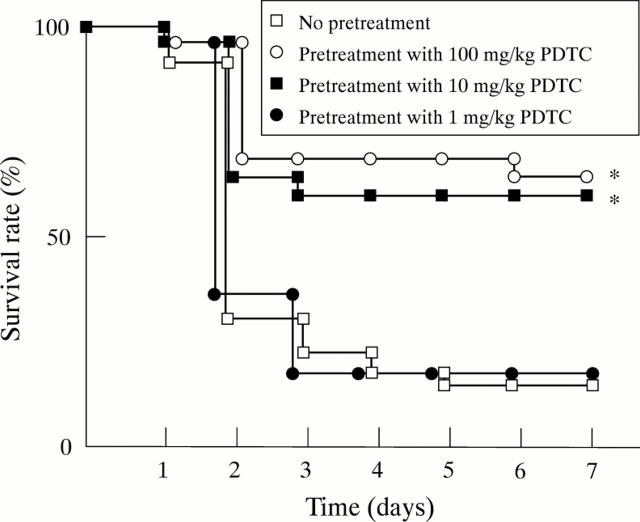
Figure 7 .
Effects of pyrrolidine dithiocarbamate (PDTC) on the mortality of the rats with taurocholate pancreatitis. PDTC was given intraperitoneally one hour before the induction of pancreatitis, and mortality was examined for seven days after the induction of pancreatitis. *p<0.05 compared with the rats not given treatment.
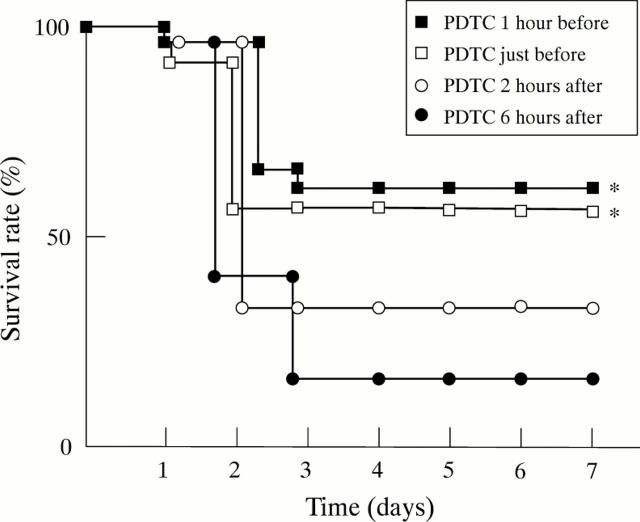
Figure 8 .
Effect of timing of pyrrolidine dithiocarbamate (PDTC) (10 mg/kg) administration on mortality. *p<0.05 compared with the rats not given treatment.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aho H. J., Koskensalo S. M., Nevalainen T. J. Experimental pancreatitis in the rat. Sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol. 1980;15(4):411–416. doi: 10.3109/00365528009181493. [DOI] [PubMed] [Google Scholar]
- Blackwell T. S., Blackwell T. R., Holden E. P., Christman B. W., Christman J. W. In vivo antioxidant treatment suppresses nuclear factor-kappa B activation and neutrophilic lung inflammation. J Immunol. 1996 Aug 15;157(4):1630–1637. [PubMed] [Google Scholar]
- Böhrer H., Qiu F., Zimmermann T., Zhang Y., Jllmer T., Männel D., Böttiger B. W., Stern D. M., Waldherr R., Saeger H. D. Role of NFkappaB in the mortality of sepsis. J Clin Invest. 1997 Sep 1;100(5):972–985. doi: 10.1172/JCI119648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceska M., Birath K., Brown B. A new and rapid method for the clinical determination of alpha-amylase activities in human serum and urine. Optimal conditions. Clin Chim Acta. 1969 Dec;26(3):437–444. doi: 10.1016/0009-8981(69)90071-0. [DOI] [PubMed] [Google Scholar]
- Denham W., Yang J., Fink G., Denham D., Carter G., Ward K., Norman J. Gene targeting demonstrates additive detrimental effects of interleukin 1 and tumor necrosis factor during pancreatitis. Gastroenterology. 1997 Nov;113(5):1741–1746. doi: 10.1053/gast.1997.v113.pm9352880. [DOI] [PubMed] [Google Scholar]
- Dunn J. A., Li C., Ha T., Kao R. L., Browder W. Therapeutic modification of nuclear factor kappa B binding activity and tumor necrosis factor-alpha gene expression during acute biliary pancreatitis. Am Surg. 1997 Dec;63(12):1036–1044. [PubMed] [Google Scholar]
- Fink G. W., Norman J. G. Intrapancreatic interleukin-1beta gene expression by specific leukocyte populations during acute pancreatitis. J Surg Res. 1996 Jun;63(1):369–373. doi: 10.1006/jsre.1996.0278. [DOI] [PubMed] [Google Scholar]
- Gianotti L., Munda R., Alexander J. W., Tchervenkov J. I., Babcock G. F. Bacterial translocation: a potential source for infection in acute pancreatitis. Pancreas. 1993 Sep;8(5):551–558. [PubMed] [Google Scholar]
- Grilli M., Chiu J. J., Lenardo M. J. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- Gross V., Andreesen R., Leser H. G., Ceska M., Liehl E., Lausen M., Farthmann E. H., Schölmerich J. Interleukin-8 and neutrophil activation in acute pancreatitis. Eur J Clin Invest. 1992 Mar;22(3):200–203. doi: 10.1111/j.1365-2362.1992.tb01826.x. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Dembinski A., Konturek P. J., Warzecha Z., Jaworek J., Gustaw P., Tomaszewska R., Stachura J. Role of platelet activating factor in pathogenesis of acute pancreatitis in rats. Gut. 1992 Sep;33(9):1268–1274. doi: 10.1136/gut.33.9.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange J. F., van Gool J., Tytgat G. N. The protective effect of a reduction in intestinal flora on mortality of acute haemorrhagic pancreatitis in the rat. Hepatogastroenterology. 1987 Feb;34(1):28–30. [PubMed] [Google Scholar]
- Lernbecher T., Müller U., Wirth T. Distinct NF-kappa B/Rel transcription factors are responsible for tissue-specific and inducible gene activation. Nature. 1993 Oct 21;365(6448):767–770. doi: 10.1038/365767a0. [DOI] [PubMed] [Google Scholar]
- Leser H. G., Gross V., Scheibenbogen C., Heinisch A., Salm R., Lausen M., Rückauer K., Andreesen R., Farthmann E. H., Schölmerich J. Elevation of serum interleukin-6 concentration precedes acute-phase response and reflects severity in acute pancreatitis. Gastroenterology. 1991 Sep;101(3):782–785. doi: 10.1016/0016-5085(91)90539-w. [DOI] [PubMed] [Google Scholar]
- Liu S. F., Ye X., Malik A. B. In vivo inhibition of nuclear factor-kappa B activation prevents inducible nitric oxide synthase expression and systemic hypotension in a rat model of septic shock. J Immunol. 1997 Oct 15;159(8):3976–3983. [PubMed] [Google Scholar]
- Lowenstein C. J., Alley E. W., Raval P., Snowman A. M., Snyder S. H., Russell S. W., Murphy W. J. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H., Henrich H., Wünsch P. H., Fischbach W., Mössner J. Role of pancreatic enzymes and their substrates in autodigestion of the pancreas. In vitro studies with isolated rat pancreatic acini. Gastroenterology. 1989 Mar;96(3):838–847. [PubMed] [Google Scholar]
- Niederau C., Liddle R. A., Ferrell L. D., Grendell J. H. Beneficial effects of cholecystokinin-receptor blockade and inhibition of proteolytic enzyme activity in experimental acute hemorrhagic pancreatitis in mice. Evidence for cholecystokinin as a major factor in the development of acute pancreatitis. J Clin Invest. 1986 Oct;78(4):1056–1063. doi: 10.1172/JCI112661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederau C., Niederau M., Borchard F., Ude K., Lüthen R., Strohmeyer G., Ferrell L. D., Grendell J. H. Effects of antioxidants and free radical scavengers in three different models of acute pancreatitis. Pancreas. 1992;7(4):486–496. doi: 10.1097/00006676-199207000-00011. [DOI] [PubMed] [Google Scholar]
- Norman J. G., Fink G. W., Franz M. G. Acute pancreatitis induces intrapancreatic tumor necrosis factor gene expression. Arch Surg. 1995 Sep;130(9):966–970. doi: 10.1001/archsurg.1995.01430090052018. [DOI] [PubMed] [Google Scholar]
- Norman J., Yang J., Fink G., Carter G., Ku G., Denham W., Livingston D. Severity and mortality of experimental pancreatitis are dependent on interleukin-1 converting enzyme (ICE). J Interferon Cytokine Res. 1997 Feb;17(2):113–118. doi: 10.1089/jir.1997.17.113. [DOI] [PubMed] [Google Scholar]
- Ryan C. M., Schmidt J., Lewandrowski K., Compton C. C., Rattner D. W., Warshaw A. L., Tompkins R. G. Gut macromolecular permeability in pancreatitis correlates with severity of disease in rats. Gastroenterology. 1993 Mar;104(3):890–895. doi: 10.1016/0016-5085(93)91027-f. [DOI] [PubMed] [Google Scholar]
- Schreck R., Meier B., Männel D. N., Dröge W., Baeuerle P. A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992 May 1;175(5):1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M. P., Aeberhard E. E., Wong V. Z., Griscavage J. M., Ignarro L. J. Pyrrolidine dithiocarbamate inhibits induction of nitric oxide synthase activity in rat alveolar macrophages. Biochem Biophys Res Commun. 1993 Mar 31;191(3):1301–1308. doi: 10.1006/bbrc.1993.1359. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Franzoso G., Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- Sobajima H., Hayakawa T., Kondo T., Shibata T., Kitagawa M., Sakai Y., Ishiguro H., Tanikawa M., Nakae Y. Effect of a new synthetic trypsin inhibitor on taurocholate-induced acute pancreatitis in rats. Pancreas. 1993 Mar;8(2):240–247. doi: 10.1097/00006676-199303000-00016. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Whisnant R., Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J Exp Med. 1993 Jun 1;177(6):1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Lin J. H., Liao H. L., Verna L., Stemerman M. B. Activation of ICAM-1 promoter by lysophosphatidylcholine: possible involvement of protein tyrosine kinases. Biochim Biophys Acta. 1997 Mar 10;1345(1):93–98. doi: 10.1016/s0005-2760(96)00169-5. [DOI] [PubMed] [Google Scholar]
- van Ooijen B., Kort W. J., Tinga C. J., Wilson J. H., Westbroek D. L. Significance of prostaglandin E2 in acute necrotising pancreatitis in rats. Gut. 1989 May;30(5):671–674. doi: 10.1136/gut.30.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]