Abstract
Background—Although many studies have investigated macromolecular uptake in the stomach and small intestine, little is known about macromolecular uptake in the colon. Aims—To investigate the mechanisms involved in the transport of large antigenically intact macromolecules across the proximal and distal colonic epithelium in the rabbit. Methods—The mucosal to serosal movement of bovine serum albumin (BSA) was examined in modified Ussing chambers under short circuited conditions. The mucosal surface was exposed to varying concentrations of BSA, and after a 50 minute equilibration period, the mucosal to serosal flux of immunologically intact BSA was determined by ELISA. Total BSA flux was determined by the transport of radiolabelled 125I-BSA. Results—Intact BSA transport in proximal and distal colonic tissue showed saturable kinetics. Intact BSA transport in the proximal and distal segment was 7% and 2% of the total 125I-BSA flux respectively. Immunologically intact BSA transport in the distal segment was significantly less than that in the proximal segment. Intact BSA transport in the proximal colon was significantly reduced following treatment with sodium fluoride, colchicine, and tetrodotoxin. Cholinergic blockade had no effect on the uptake of intact BSA. Conclusion—The findings indicate that the transport of intact macromolecules across the proximal and distal large intestine is a saturable process. Further, intact BSA transport in the proximal colon is an energy dependent process that utilises microtubules and is regulated by the enteric nervous system.
Keywords: colon; transport; protein
Full Text
The Full Text of this article is available as a PDF (167.8 KB).
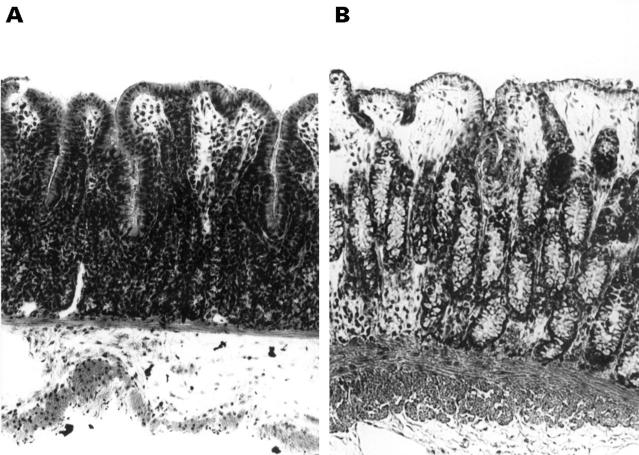
Figure 1 .
Representative micrographs of stripped colonic tissue obtained 80 minutes after mounting in the Ussing chamber. (A) proximal colon; (B) distal colon. Original magnification × 400.
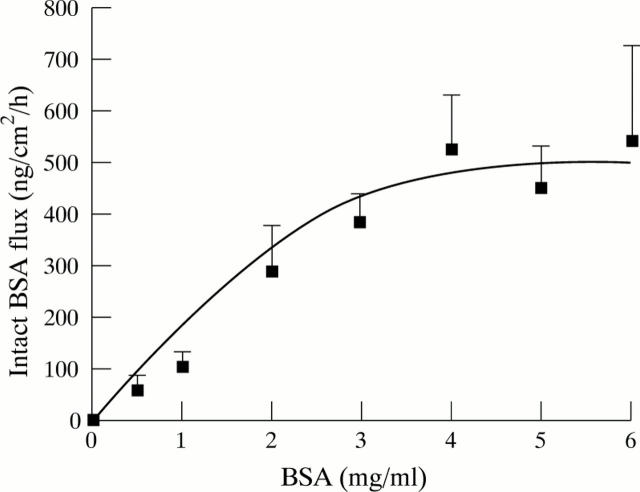
Figure 2 .
Dose response curve for intact BSA transport in the proximal colon. Data represent the mean (SEM) of at least three tissues at each concentration.
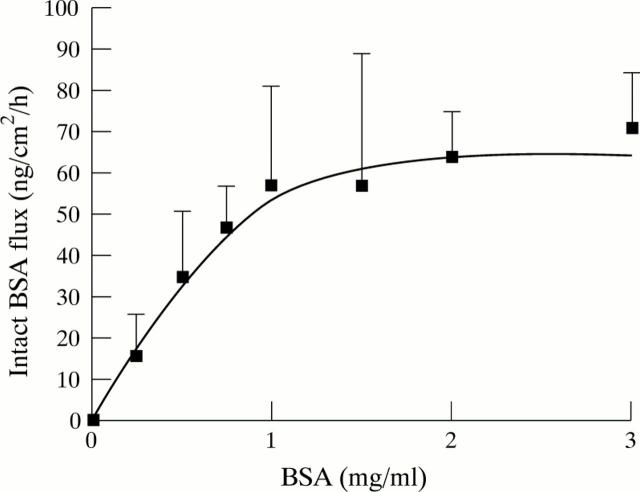
Figure 3 .
Dose response curve for intact BSA transport in the distal colon. Data represent the mean (SEM) of at least three tissues at each concentration.
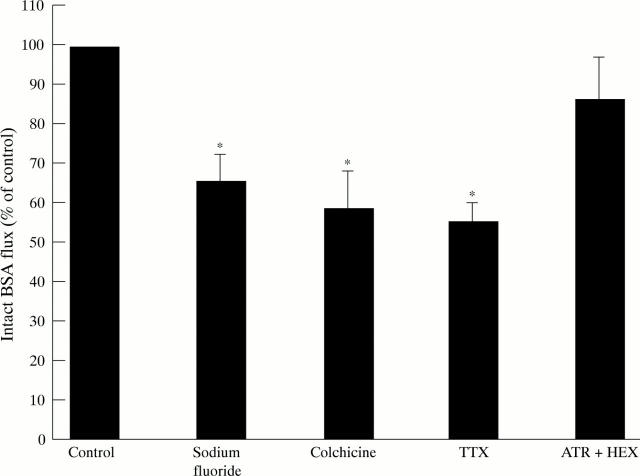
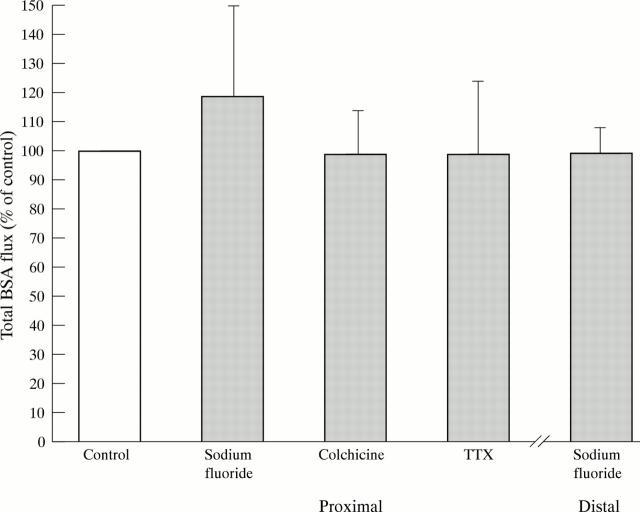
Figure 4 .
Metabolic inhibition and neural regulation of intact BSA transport in proximal colonic tissue. NaF (n=6), colchicine (n=10), TTX (n=9), ATR + HEX (n=4), controls (n=the number in each experimental group). All measurements were performed in paired tissue. *p<0.05.
Figure 5 .
Metabolic, microtubule, and neural inhibition of total (intact and degraded) BSA. n=4 for all groups. All measurements were performed in paired tissue.
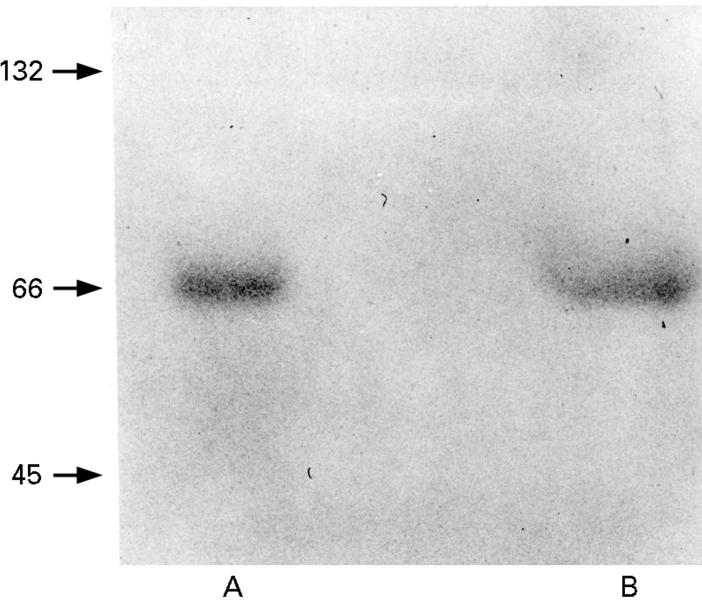
Figure 6 .
Immunoblot of BSA collected (A) fresh (immediately after preparation), and (B) from the serosal chamber after 80 minutes. Immunologically intact BSA is visualised as a single 66.2 kDa band in the sample obtained from (B).
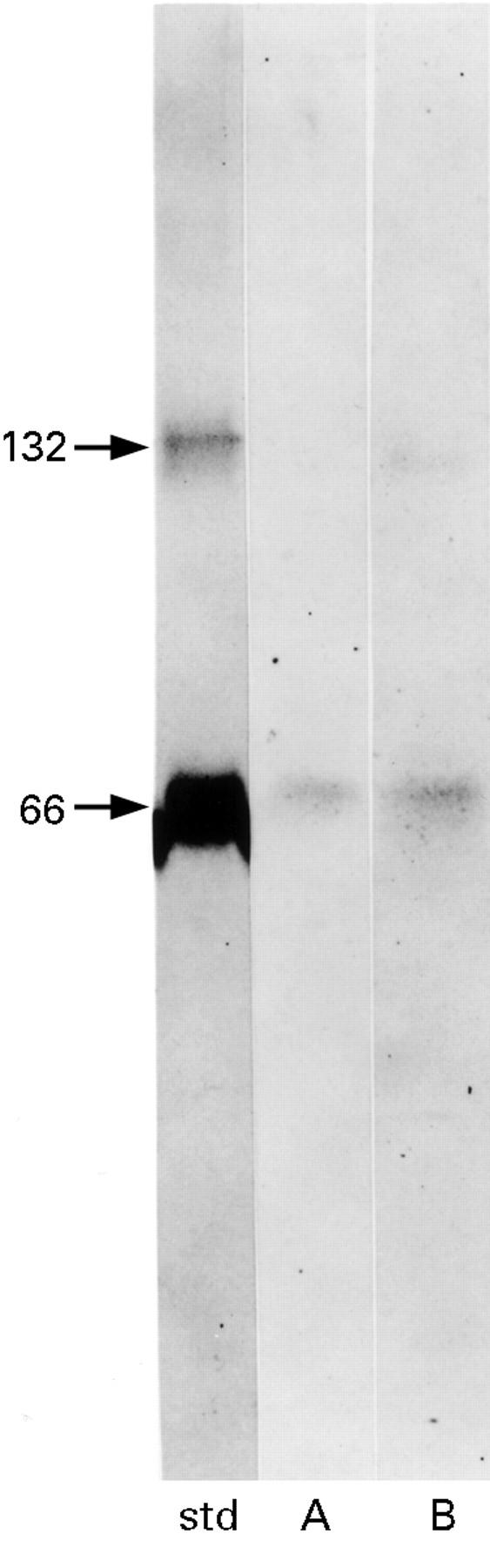
Figure 7 .
Autoradiograph of mucosal solution obtained at 80 minutes containing 125I-BSA (A) and freshly prepared 125I-BSA (B). BSA was not degraded prior to transport across the tissue and remained conjugated to 125I.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander H. L., Shirley K., Allen D. THE ROUTE OF INGESTED EGG WHITE TO THE SYSTEMIC CIRCULATION. J Clin Invest. 1936 Mar;15(2):163–167. doi: 10.1172/JCI100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berin M. C., Kiliaan A. J., Yang P. C., Groot J. A., Taminiau J. A., Perdue M. H. Rapid transepithelial antigen transport in rat jejunum: impact of sensitization and the hypersensitivity reaction. Gastroenterology. 1997 Sep;113(3):856–864. doi: 10.1016/s0016-5085(97)70180-x. [DOI] [PubMed] [Google Scholar]
- Bern M. J., Sturbaum C. W., Karayalcin S. S., Berschneider H. M., Wachsman J. T., Powell D. W. Immune system control of rat and rabbit colonic electrolyte transport. Role of prostaglandins and enteric nervous system. J Clin Invest. 1989 Jun;83(6):1810–1820. doi: 10.1172/JCI114086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlsma P. B., Kiliaan A. J., Scholten G., Heyman M., Groot J. A., Taminiau J. A. Carbachol, but not forskolin, increases mucosal-to-serosal transport of intact protein in rat ileum in vitro. Am J Physiol. 1996 Jul;271(1 Pt 1):G147–G155. doi: 10.1152/ajpgi.1996.271.1.G147. [DOI] [PubMed] [Google Scholar]
- Catto-Smith A. G., Patrick M. K., Hardin J. A., Gall D. G. Intestinal anaphylaxis in the rat: mediators responsible for the ion transport abnormalities. Agents Actions. 1989 Nov;28(3-4):185–191. doi: 10.1007/BF01967399. [DOI] [PubMed] [Google Scholar]
- Curtis G. H., Gall D. G. Macromolecular transport by rat gastric mucosa. Am J Physiol. 1992 Jun;262(6 Pt 1):G1033–G1040. doi: 10.1152/ajpgi.1992.262.6.G1033. [DOI] [PubMed] [Google Scholar]
- Curtis G. H., MacNaughton W. K., Gall D. G. Effect of cyclooxygenase inhibition on macromolecular transport in rat gastric mucosa. Am J Physiol. 1993 Dec;265(6 Pt 1):G1135–G1140. doi: 10.1152/ajpgi.1993.265.6.G1135. [DOI] [PubMed] [Google Scholar]
- Ferry D. M., Butt T. J., Broom M. F., Hunter J., Chadwick V. S. Bacterial chemotactic oligopeptides and the intestinal mucosal barrier. Gastroenterology. 1989 Jul;97(1):61–67. doi: 10.1016/0016-5085(89)91416-9. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Heyman M., Crain-Denoyelle A. M., Corthier G., Morgat J. L., Desjeux J. F. Postnatal development of protein absorption in conventional and germ-free mice. Am J Physiol. 1986 Sep;251(3 Pt 1):G326–G331. doi: 10.1152/ajpgi.1986.251.3.G326. [DOI] [PubMed] [Google Scholar]
- Heyman M., Crain-Denoyelle A. M., Nath S. K., Desjeux J. F. Quantification of protein transcytosis in the human colon carcinoma cell line CaCo-2. J Cell Physiol. 1990 May;143(2):391–395. doi: 10.1002/jcp.1041430225. [DOI] [PubMed] [Google Scholar]
- Heyman M., Desjeux J. F. Significance of intestinal food protein transport. J Pediatr Gastroenterol Nutr. 1992 Jul;15(1):48–57. [PubMed] [Google Scholar]
- Heyman M., Ducroc R., Desjeux J. F., Morgat J. L. Horseradish peroxidase transport across adult rabbit jejunum in vitro. Am J Physiol. 1982 Jun;242(6):G558–G564. doi: 10.1152/ajpgi.1982.242.6.G558. [DOI] [PubMed] [Google Scholar]
- Heyman M., Grasset E., Ducroc R., Desjeux J. F. Antigen absorption by the jejunal epithelium of children with cow's milk allergy. Pediatr Res. 1988 Aug;24(2):197–202. doi: 10.1203/00006450-198808000-00012. [DOI] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Hollander D., Vadheim C. M., Brettholz E., Petersen G. M., Delahunty T., Rotter J. I. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986 Dec;105(6):883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- Isolauri E., Gotteland M., Heyman M., Pochart P., Desjeux J. F. Antigen absorption in rabbit bacterial diarrhea (RDEC-1). In vitro modifications in ileum and Peyer's patches. Dig Dis Sci. 1990 Mar;35(3):360–366. doi: 10.1007/BF01537415. [DOI] [PubMed] [Google Scholar]
- Keljo D. J., Butler D. G., Hamilton J. R. Altered jejunal permeability to macromolecules during viral enteritis in the piglet. Gastroenterology. 1985 Apr;88(4):998–1004. doi: 10.1016/S0016-5085(85)80020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keljo D. J., Hamilton J. R. Quantitative determination of macromolecular transport rate across intestinal Peyer's patches. Am J Physiol. 1983 Jun;244(6):G637–G644. doi: 10.1152/ajpgi.1983.244.6.G637. [DOI] [PubMed] [Google Scholar]
- Kimm M. H., Curtis G. H., Hardin J. A., Gall D. G. Transport of bovine serum albumin across rat jejunum: role of the enteric nervous system. Am J Physiol. 1994 Feb;266(2 Pt 1):G186–G193. doi: 10.1152/ajpgi.1994.266.2.G186. [DOI] [PubMed] [Google Scholar]
- Kimm M. H., Hardin J. A., Gall D. G. The role of nitric oxide in the regulation of macromolecular transport in rat jejunum. J Physiol. 1996 Jan 1;490(Pt 1):243–248. doi: 10.1113/jphysiol.1996.sp021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimm M. H., Hardin J. A., Gall D. G. Transport of albumin into the intestinal lumen of the rat. Can J Physiol Pharmacol. 1997 Mar;75(3):193–198. [PubMed] [Google Scholar]
- Kosecka U., Marshall J. S., Crowe S. E., Bienenstock J., Perdue M. H. Pertussis toxin stimulates hypersensitivity and enhances nerve-mediated antigen uptake in rat intestine. Am J Physiol. 1994 Nov;267(5 Pt 1):G745–G753. doi: 10.1152/ajpgi.1994.267.5.G745. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lundin S., Vilhardt H. Absorption of 1-deamino-8-D-arginine vasopressin from different regions of the gastrointestinal tract in rabbits. Acta Endocrinol (Copenh) 1986 Jul;112(3):457–460. doi: 10.1530/acta.0.1120457. [DOI] [PubMed] [Google Scholar]
- MacQueen G., Marshall J., Perdue M., Siegel S., Bienenstock J. Pavlovian conditioning of rat mucosal mast cells to secrete rat mast cell protease II. Science. 1989 Jan 6;243(4887):83–85. doi: 10.1126/science.2911721. [DOI] [PubMed] [Google Scholar]
- Machida H. M., Catto Smith A. G., Gall D. G., Trevenen C., Scott R. B. Allergic colitis in infancy: clinical and pathologic aspects. J Pediatr Gastroenterol Nutr. 1994 Jul;19(1):22–26. doi: 10.1097/00005176-199407000-00004. [DOI] [PubMed] [Google Scholar]
- Magnusson K. E., Dahlgren C., Sjölander A. Effect of N-formylated methionyl-phenylalanine (FMP) and methionyl-leucyl-phenylalanine (FMLP) on gut permeability. A model of local inflammatory process. Inflammation. 1985 Dec;9(4):365–373. doi: 10.1007/BF00916336. [DOI] [PubMed] [Google Scholar]
- Malin M., Isolauri E., Pikkarainen P., Karikoski R., Isolauri J. Enhanced absorption of macromolecules. A secondary factor in Crohn's disease. Dig Dis Sci. 1996 Jul;41(7):1423–1428. doi: 10.1007/BF02088568. [DOI] [PubMed] [Google Scholar]
- Marcon-Genty D., Tome D., Kheroua O., Dumontier A. M., Heyman M., Desjeux J. F. Transport of beta-lactoglobulin across rabbit ileum in vitro. Am J Physiol. 1989 Jun;256(6 Pt 1):G943–G948. doi: 10.1152/ajpgi.1989.256.6.G943. [DOI] [PubMed] [Google Scholar]
- Newson B., Dahlström A., Enerbäck L., Ahlman H. Suggestive evidence for a direct innervation of mucosal mast cells. Neuroscience. 1983 Oct;10(2):565–570. doi: 10.1016/0306-4522(83)90153-7. [DOI] [PubMed] [Google Scholar]
- Ramage J. K., Stanisz A., Scicchitano R., Hunt R. H., Perdue M. H. Effect of immunologic reactions on rat intestinal epithelium. Correlation of increased permeability to chromium 51-labeled ethylenediaminetetraacetic acid and ovalbumin during acute inflammation and anaphylaxis. Gastroenterology. 1988 Jun;94(6):1368–1375. [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Stahl P., Schwartz A. L. Receptor-mediated endocytosis. J Clin Invest. 1986 Mar;77(3):657–662. doi: 10.1172/JCI112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead R. H., Tomioka M., Quinonez G., Simon G. T., Felten S. Y., Bienenstock J. Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proc Natl Acad Sci U S A. 1987 May;84(9):2975–2979. doi: 10.1073/pnas.84.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H. C., Parrott M. V. The induction of tolerance to a soluble protein antigen by oral administration. Immunology. 1974 Oct;27(4):631–639. [PMC free article] [PubMed] [Google Scholar]
- Videla S., Vilaseca J., Guarner F., Salas A., Treserra F., Crespo E., Antolín M., Malagelada J. R. Role of intestinal microflora in chronic inflammation and ulceration of the rat colon. Gut. 1994 Aug;35(8):1090–1097. doi: 10.1136/gut.35.8.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W. A., Cornell R., Davenport L. M., Isselbacher K. J. Macromolecular absorption. Mechanism of horseradish peroxidase uptake and transport in adult and neonatal rat intestine. J Cell Biol. 1972 Aug;54(2):195–205. doi: 10.1083/jcb.54.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshaw A. L., Walker W. A., Cornell R., Isselbacher K. J. Small intestinal permeability to macromolecules. Transmission of horseradish peroxidase into mesenteric lymph and portal blood. Lab Invest. 1971 Dec;25(6):675–684. [PubMed] [Google Scholar]
- von Ritter C., Sekizuka E., Grisham M. B., Granger D. N. The chemotactic peptide N-formyl methionyl-leucyl-phenylalanine increases mucosal permeability in the distal ileum of the rat. Gastroenterology. 1988 Sep;95(3):651–656. doi: 10.1016/s0016-5085(88)80011-8. [DOI] [PubMed] [Google Scholar]