Abstract
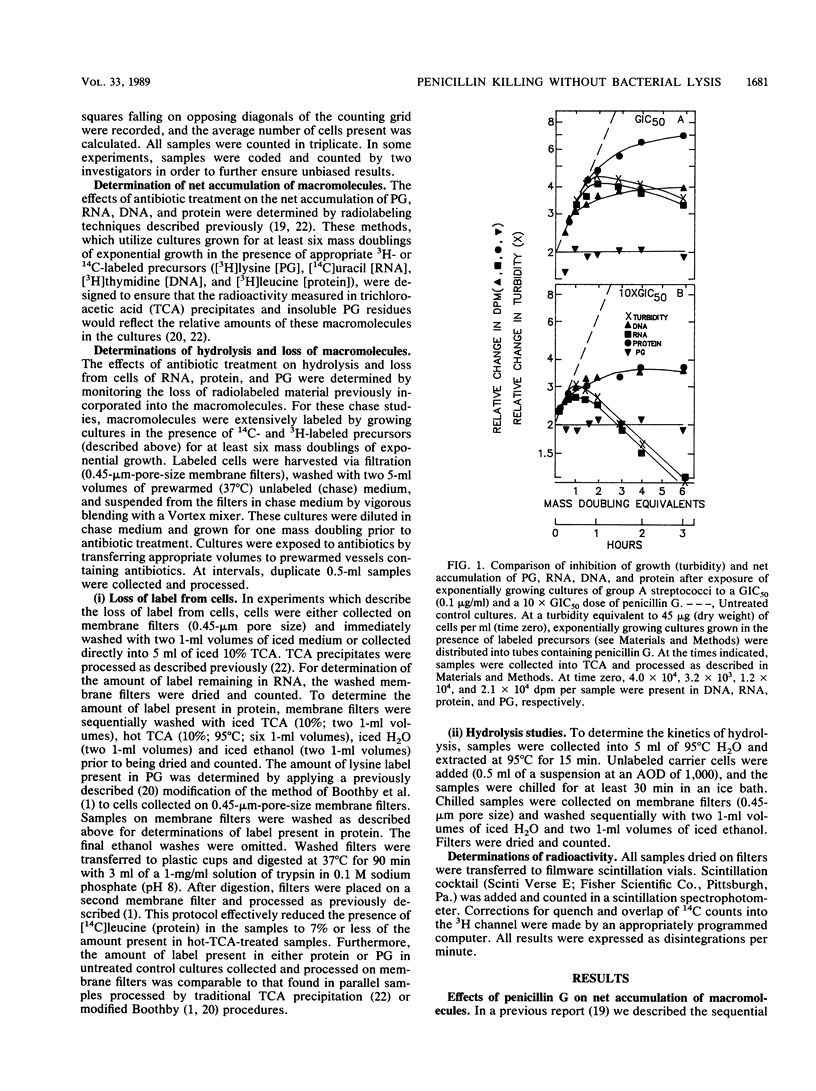
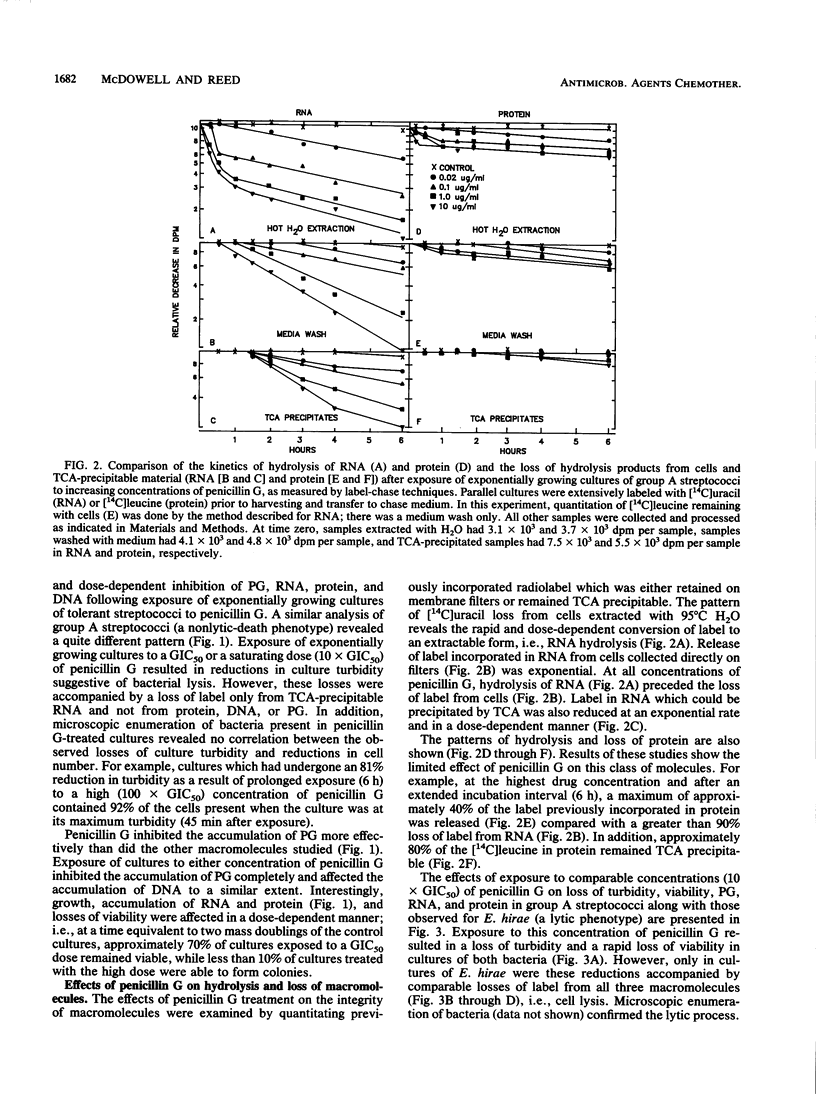
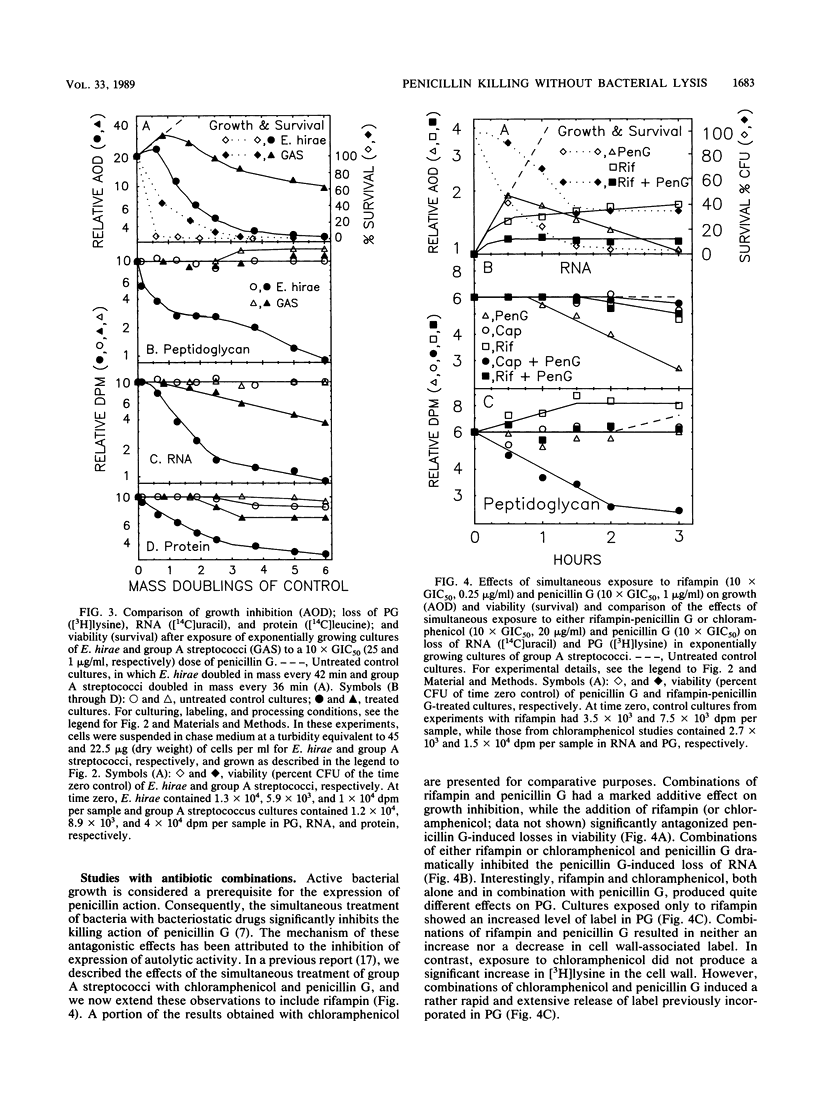
Exposure of group A streptococci (a nonlytic-death phenotype) to benzylpenicillin (penicillin G) produced a dose-dependent, rapid, and extensive hydrolysis of total cellular RNA, with the subsequent loss of hydrolysis products from the cell. This loss of RNA correlated well with loss of viability and was not accompanied by solubilization of the cell wall or comparable losses of either protein or DNA. Simultaneous treatment with penicillin G and either chloramphenicol or rifampin resulted in reduced levels of killing and the complete inhibition of RNA loss. These findings define a new mechanism of penicillin G-induced killing in the absence of cell wall disruption and suggest a basis for drug-induced antagonism of penicillin G-mediated nonlytic death.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boothby D., Daneo-Moore L., Shockman G. D. A rapid, guantitative, and selective estimation of radioactively labeled peptidoglycan in gram-positive bacteria. Anal Biochem. 1971 Dec;44(2):645–653. doi: 10.1016/0003-2697(71)90255-7. [DOI] [PubMed] [Google Scholar]
- Cozens R. M., Tuomanen E., Tosch W., Zak O., Suter J., Tomasz A. Evaluation of the bactericidal activity of beta-lactam antibiotics on slowly growing bacteria cultured in the chemostat. Antimicrob Agents Chemother. 1986 May;29(5):797–802. doi: 10.1128/aac.29.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann L., Tomasz A. Penicillin-resistant and penicillin-tolerant mutants of group A Streptococci. Antimicrob Agents Chemother. 1982 Jul;22(1):128–136. doi: 10.1128/aac.22.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Daneo-Moore L., Boothby D., Shockman G. D. Effect of inhibition of deoxyribonucleic acid and protein synthesis on the direction of cell wall growth in Streptococcus faecalis. J Bacteriol. 1974 May;118(2):681–692. doi: 10.1128/jb.118.2.681-692.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Early changes in the ultrastructure of Streptococcus faecalis after amino acid starvation. J Bacteriol. 1970 Jul;103(1):244–253. doi: 10.1128/jb.103.1.244-253.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Tolerant response of Streptococcus sanguis to beta-lactams and other cell wall inhibitors. Antimicrob Agents Chemother. 1977 May;11(5):888–896. doi: 10.1128/aac.11.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAWETZ E., GUNNISON J. B., SPECK R. S., COLEMAN V. R. Studies on antibiotic synergism and antagonism; the interference of chloramphenicol with the action of penicillin. AMA Arch Intern Med. 1951 Mar;87(3):349–359. doi: 10.1001/archinte.1951.03810030022002. [DOI] [PubMed] [Google Scholar]
- Kitano K., Tomasz A. Escherichia coli mutants tolerant to beta-lactam antibiotics. J Bacteriol. 1979 Dec;140(3):955–963. doi: 10.1128/jb.140.3.955-963.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusser W., Ishiguro E. E. Lysis of nongrowing Escherichia coli by combinations of beta-lactam antibiotics and inhibitors of ribosome function. Antimicrob Agents Chemother. 1986 Mar;29(3):451–455. doi: 10.1128/aac.29.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. H., Tomasz A. Penicillin tolerance in multiply drug-resistant natural isolates of Streptococcus pneumoniae. J Infect Dis. 1985 Aug;152(2):365–372. doi: 10.1093/infdis/152.2.365. [DOI] [PubMed] [Google Scholar]
- Lorian V., Sabath L. D., Simionescu M., Watson D. W. Decrease in ribosomal density of Proteus mirabilis exposed to subinhibitory concentrations of ampicillin or cephalothin. Proc Soc Exp Biol Med. 1975 Jul;149(3):731–735. doi: 10.3181/00379727-149-38888. [DOI] [PubMed] [Google Scholar]
- Mattingly S. J., Daneo-Moore L., Shockman G. D. Factors regulating cell wall thickening and intracellular iodophilic polysaccharide storage in Streptococcus mutans. Infect Immun. 1977 Jun;16(3):967–973. doi: 10.1128/iai.16.3.967-973.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly S. J., Dipersio J. R., Higgins M. L., Shockman G. D. Unbalanced growth and macromolecular synthesis in Streptococcus mutans FA-1. Infect Immun. 1976 Mar;13(3):941–948. doi: 10.1128/iai.13.3.941-948.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell T. D., Lemanski C. L. Absence of autolytic activity (peptidoglycan nicking) in penicillin-induced nonlytic death in a group A streptococcus. J Bacteriol. 1988 Apr;170(4):1783–1788. doi: 10.1128/jb.170.4.1783-1788.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell T. D., McCurdy W., Reed K. E. Talk-back regulation: a regulatory response to the inhibitions of cell surface growth. Microbios. 1989;57(232-233):187–204. [PubMed] [Google Scholar]
- Mychajlonka M., McDowell T. D., Shockman G. D. Conservation of cell wall peptidoglycan by strains of Streptococcus mutans and Streptococcus sanguis. Infect Immun. 1980 Apr;28(1):65–73. doi: 10.1128/iai.28.1.65-73.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychajlonka M., McDowell T. D., Shockman G. D. Inhibition of peptidoglycan, ribonucleic acid, and protein synthesis in tolerant strains of Streptococcus mutans. Antimicrob Agents Chemother. 1980 Apr;17(4):572–582. doi: 10.1128/aac.17.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESTIDGE L. S., PARDEE A. B. Induction of bacterial lysis by penicillin. J Bacteriol. 1957 Jul;74(1):48–59. doi: 10.1128/jb.74.1.48-59.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G. S., Shockman G. D., Daneo-Moore L. Balanced macromolecular biosynthesis in "protoplasts" of Streptococcus faecalis. J Bacteriol. 1971 Mar;105(3):710–717. doi: 10.1128/jb.105.3.710-717.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol. 1979;33:113–137. doi: 10.1146/annurev.mi.33.100179.000553. [DOI] [PubMed] [Google Scholar]
- Wegener W. S., Hebeler B. H., Morse S. A. Cell envelope of Neisseria gonorrhoeae: penicillin enhancement of peptidoglycan hydrolysis. Infect Immun. 1977 Dec;18(3):717–725. doi: 10.1128/iai.18.3.717-725.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Kessler R. E. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun. 1980 Feb;27(2):444–448. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


