Abstract
Background—Inflammatory bowel diseases (IBD) are characterised by an intense infiltration of leucocytes that is mediated by adhesion molecules expressed on the surface of activated endothelial cells. Aims—To determine whether drugs used in the treatment of IBD, specifically dexamethasone (DEX), 5-aminosalicylic acid (5-ASA), methotrexate (MTX), and 6-mercaptopurine (6-MP), alter the expression of endothelial cell adhesion molecules (ECAMs). Methods—The expression of P-selectin, E-selectin, intercellular adhesion molecule 1 (ICAM-1), and vascular CAM 1(VCAM-1) in different vascular beds of C57Bl/6J mice was measured using the dual radiolabelled monoclonal antibody technique. Results—Lipopolysaccharide (LPS) elicited a profound increase in the expression of all ECAMs in the mesentery, small intestine, caecum, and distal colon. The LPS induced increase in CAM expression was not significantly affected by prior treatment with either MTX or 6-MP. However, pretreatment with either DEX or 5-ASA significantly attenuated LPS induced increases in expression of P- and E-selectin, and VCAM-1 in the majority of tissues evaluated. DEX also blunted the LPS induced increase in ICAM-1 expression in the caecum and distal colon. DEX, but not 5-ASA, largely abolished the rise in plasma tumour necrosis factor α elicited by LPS. Conclusions—These findings suggest that DEX and 5-ASA may exert their beneficial therapeutic action in IBD, at least in part, by inhibiting the expression of ECAMs which mediate leucocyte adhesion and transmigration in the microvasculature.
Keywords: P-selectin; E-selectin; intercellular adhesion molecule 1; vascular cell adhesion molecule 1; dexamethasone; 5-aminosalicylic acid
Full Text
The Full Text of this article is available as a PDF (186.3 KB).
Figure 1 .

Effect of anti-inflammatory drugs on the expression of P-selectin in (A) mesentery, (B) small intestine, (C) caecum, and (D) distal colon. The numbers of animals in each experimental group was as follows: control, 5; LPS + control, 7; LPS + 5-ASA, 5; LPS + DEX, 5; LPS + MTX, 5; LPS + 6-MP, 5. *p<0.05 versus LPS + untreated.
Figure 2 .

Effect of anti-inflammatory drugs on the expression of E-selectin in (A) mesentery, (B) small intestine, (C) caecum, and (D) distal colon. The numbers of animals in each experimental group were as follows: control, 5; LPS + control, 7; LPS + 5-ASA, 5; LPS + DEX, 5; LPS + MTX, 5; LPS + 6-MP, 5. *p<0.05 versus LPS + untreated.
Figure 3 .
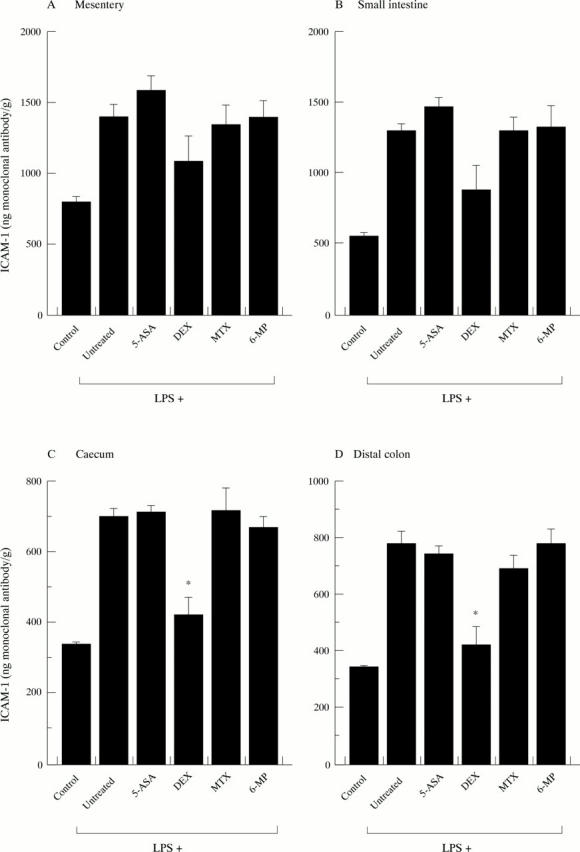
Effect of anti-inflammatory drugs on the expression of ICAM-1 in (A) mesentery, (B) small intestine, (C) caecum, and (D) distal colon. The numbers of animals in each experimental group were as follows: control, 5; LPS + control, 7; LPS + 5-ASA, 5; LPS + DEX, 5; LPS + MTX, 5; LPS + 6-MP, 5. *p<0.05 versus LPS + untreated.
Figure 4 .

Effect of anti-inflammatory drugs on the expression of VCAM-1 in (A) mesentery, (B) small intestine, (C) caecum, and (D) distal colon. The numbers of animals in each experimental group were as follows: control, 5; LPS + control, 7; LPS + 5-ASA, 5; LPS + DEX, 5; LPS + MTX, 5; LPS + 6-MP, 5. *p<0.05 versus LPS + untreated.
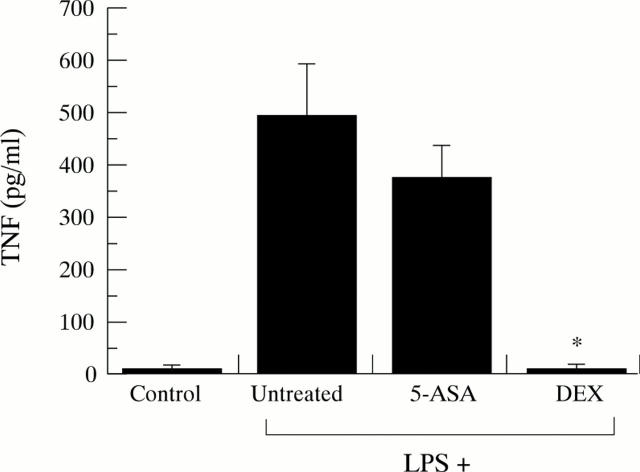
Figure 5 .
Effect of 5-ASA and DEX on plasma TNF-α concentrations in stimulated animals. The numbers of animals in each experimental group was as follows: control, 5; LPS + control, 5; LPS + 5-ASA, 5; LPS + DEX, 5. *p<0.05 versus LPS + untreated.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asako H., Kubes P., Baethge B. A., Wolf R. E., Granger D. N. Colchicine and methotrexate reduce leukocyte adherence and emigration in rat mesenteric venules. Inflammation. 1992 Feb;16(1):45–56. doi: 10.1007/BF00917514. [DOI] [PubMed] [Google Scholar]
- Aziz K. E., Wakefield D. Modulation of endothelial cell expression of ICAM-1, E-selectin, and VCAM-1 by beta-estradiol, progesterone, and dexamethasone. Cell Immunol. 1996 Jan 10;167(1):79–85. doi: 10.1006/cimm.1996.0010. [DOI] [PubMed] [Google Scholar]
- Bonner G. F. Current medical therapy for inflammatory bowel disease. South Med J. 1996 Jun;89(6):556–566. doi: 10.1097/00007611-199606000-00002. [DOI] [PubMed] [Google Scholar]
- Brostjan C., Anrather J., Csizmadia V., Natarajan G., Winkler H. Glucocorticoids inhibit E-selectin expression by targeting NF-kappaB and not ATF/c-Jun. J Immunol. 1997 Apr 15;158(8):3836–3844. [PubMed] [Google Scholar]
- Collins T. Endothelial nuclear factor-kappa B and the initiation of the atherosclerotic lesion. Lab Invest. 1993 May;68(5):499–508. [PubMed] [Google Scholar]
- Cronstein B. N., Kimmel S. C., Levin R. I., Martiniuk F., Weissmann G. A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):9991–9995. doi: 10.1073/pnas.89.21.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Naime D., Ostad E. The antiinflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest. 1993 Dec;92(6):2675–2682. doi: 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJoy S. Q., Jeyaseelan R., Sr, Torley L. W., Schow S. R., Wick M. M., Kerwar S. S. Attenuation of interleukin 2-induced pulmonary vascular leak syndrome by low doses of oral methotrexate. Cancer Res. 1995 Nov 1;55(21):4929–4935. [PubMed] [Google Scholar]
- Detmar M., Tenorio S., Hettmannsperger U., Ruszczak Z., Orfanos C. E. Cytokine regulation of proliferation and ICAM-1 expression of human dermal microvascular endothelial cells in vitro. J Invest Dermatol. 1992 Feb;98(2):147–153. doi: 10.1111/1523-1747.ep12555746. [DOI] [PubMed] [Google Scholar]
- Eppihimer M. J., Russell J., Anderson D. C., Wolitzky B. A., Granger D. N. Endothelial cell adhesion molecule expression in gene-targeted mice. Am J Physiol. 1997 Oct;273(4 Pt 2):H1903–H1908. doi: 10.1152/ajpheart.1997.273.4.H1903. [DOI] [PubMed] [Google Scholar]
- Eppihimer M. J., Wolitzky B., Anderson D. C., Labow M. A., Granger D. N. Heterogeneity of expression of E- and P-selectins in vivo. Circ Res. 1996 Sep;79(3):560–569. doi: 10.1161/01.res.79.3.560. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick L. R., Bostwick J. S., Renzetti M., Pendleton R. G., Decktor D. L. Antiinflammatory effects of various drugs on acetic acid induced colitis in the rat. Agents Actions. 1990 Jun;30(3-4):393–402. doi: 10.1007/BF01966304. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Granger D. N., Kubes P. The microcirculation and inflammation: modulation of leukocyte-endothelial cell adhesion. J Leukoc Biol. 1994 May;55(5):662–675. [PubMed] [Google Scholar]
- Greenfield S. M., Punchard N. A., Teare J. P., Thompson R. P. Review article: the mode of action of the aminosalicylates in inflammatory bowel disease. Aliment Pharmacol Ther. 1993 Aug;7(4):369–383. doi: 10.1111/j.1365-2036.1993.tb00110.x. [DOI] [PubMed] [Google Scholar]
- Haraldsen G., Kvale D., Lien B., Farstad I. N., Brandtzaeg P. Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human microvascular endothelial cells. J Immunol. 1996 Apr 1;156(7):2558–2565. [PubMed] [Google Scholar]
- Henninger D. D., Panés J., Eppihimer M., Russell J., Gerritsen M., Anderson D. C., Granger D. N. Cytokine-induced VCAM-1 and ICAM-1 expression in different organs of the mouse. J Immunol. 1997 Feb 15;158(4):1825–1832. [PubMed] [Google Scholar]
- Henseleit U., Steinbrink K., Sunderkötter C., Goebeler M., Roth J., Sorg C. Expression of murine VCAM-1 in vitro and in different models of inflammation in vivo: correlation with immigration of monocytes. Exp Dermatol. 1994 Dec;3(6):249–256. [PubMed] [Google Scholar]
- Hettmannsperger U., Tenorio S., Orfanos C. E., Detmar M. Corticosteroids induce proliferation but do not influence TNF- or IL-1 beta-induced ICAM-1 expression of human dermal microvascular endothelial cells in vitro. Arch Dermatol Res. 1993;285(6):347–351. doi: 10.1007/BF00371835. [DOI] [PubMed] [Google Scholar]
- Hibi T., Iwao Y., Yajima T., Inoue N., Ueno Y., Takaishi H., Watanabe M., Ishii H. Immunosuppressive agents in the treatment of Crohn's disease and ulcerative colitis. J Gastroenterol. 1995 Nov;30 (Suppl 8):121–123. [PubMed] [Google Scholar]
- James S. P., Klapproth J. M. Major pathways of mucosal immunity and inflammation: cell activation, cytokine production and the role of bacterial factors. Aliment Pharmacol Ther. 1996;10 (Suppl 2):1–9. doi: 10.1046/j.1365-2036.1996.22164000.x. [DOI] [PubMed] [Google Scholar]
- Klemm P., Harris H. J., Perretti M. Effect of rolipram in a murine model of acute inflammation: comparison with the corticoid dexamethasone. Eur J Pharmacol. 1995 Jul 25;281(1):69–74. doi: 10.1016/0014-2999(95)00232-a. [DOI] [PubMed] [Google Scholar]
- Koizumi M., King N., Lobb R., Benjamin C., Podolsky D. K. Expression of vascular adhesion molecules in inflammatory bowel disease. Gastroenterology. 1992 Sep;103(3):840–847. doi: 10.1016/0016-5085(92)90015-q. [DOI] [PubMed] [Google Scholar]
- Kojouharoff G., Hans W., Obermeier F., Männel D. N., Andus T., Schölmerich J., Gross V., Falk W. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin Exp Immunol. 1997 Feb;107(2):353–358. doi: 10.1111/j.1365-2249.1997.291-ce1184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P., Kanwar S. Histamine induces leukocyte rolling in post-capillary venules. A P-selectin-mediated event. J Immunol. 1994 Apr 1;152(7):3570–3577. [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Neutrophils roll on E-selectin. J Immunol. 1993 Dec 1;151(11):6338–6346. [PubMed] [Google Scholar]
- Ma L., Raycroft L., Asa D., Anderson D. C., Geng J. G. A sialoglycoprotein from human leukocytes functions as a ligand for P-selectin. J Biol Chem. 1994 Nov 4;269(44):27739–27746. [PubMed] [Google Scholar]
- Malizia G., Calabrese A., Cottone M., Raimondo M., Trejdosiewicz L. K., Smart C. J., Oliva L., Pagliaro L. Expression of leukocyte adhesion molecules by mucosal mononuclear phagocytes in inflammatory bowel disease. Gastroenterology. 1991 Jan;100(1):150–159. doi: 10.1016/0016-5085(91)90595-c. [DOI] [PubMed] [Google Scholar]
- McCafferty D. M., Granger D. N., Wallace J. L. Indomethacin-induced gastric injury and leukocyte adherence in arthritic versus healthy rats. Gastroenterology. 1995 Oct;109(4):1173–1180. doi: 10.1016/0016-5085(95)90576-6. [DOI] [PubMed] [Google Scholar]
- Meyer M., Schreck R., Baeuerle P. A. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 1993 May;12(5):2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Medina K., Ishihara K., Kimoto M., Auerbach R., Kincade P. W. A VCAM-like adhesion molecule on murine bone marrow stromal cells mediates binding of lymphocyte precursors in culture. J Cell Biol. 1991 Aug;114(3):557–565. doi: 10.1083/jcb.114.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Weissman I. L., Greenberger J. S., Kincade P. W. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med. 1991 Mar 1;173(3):599–607. doi: 10.1084/jem.173.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noronha-Blob L., Lowe V. C., Muhlhauser R. O., Burch R. M. NPC 15669, an inhibitor of neutrophil recruitment, is efficacious in acetic acid-induced colitis in rats. Gastroenterology. 1993 Apr;104(4):1021–1029. doi: 10.1016/0016-5085(93)90269-i. [DOI] [PubMed] [Google Scholar]
- Norton C. R., Rumberger J. M., Burns D. K., Wolitzky B. A. Characterization of murine E-selectin expression in vitro using novel anti-mouse E-selectin monoclonal antibodies. Biochem Biophys Res Commun. 1993 Aug 31;195(1):250–258. doi: 10.1006/bbrc.1993.2037. [DOI] [PubMed] [Google Scholar]
- Panés J., Granger D. N. Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology. 1998 May;114(5):1066–1090. doi: 10.1016/s0016-5085(98)70328-2. [DOI] [PubMed] [Google Scholar]
- Panés J., Perry M. A., Anderson D. C., Manning A., Leone B., Cepinskas G., Rosenbloom C. L., Miyasaka M., Kvietys P. R., Granger D. N. Regional differences in constitutive and induced ICAM-1 expression in vivo. Am J Physiol. 1995 Dec;269(6 Pt 2):H1955–H1964. doi: 10.1152/ajpheart.1995.269.6.H1955. [DOI] [PubMed] [Google Scholar]
- Patel K. D., Moore K. L., Nollert M. U., McEver R. P. Neutrophils use both shared and distinct mechanisms to adhere to selectins under static and flow conditions. J Clin Invest. 1995 Oct;96(4):1887–1896. doi: 10.1172/JCI118234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picarella D., Hurlbut P., Rottman J., Shi X., Butcher E., Ringler D. J. Monoclonal antibodies specific for beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of scid mice reconstituted with CD45RBhigh CD4+ T cells. J Immunol. 1997 Mar 1;158(5):2099–2106. [PubMed] [Google Scholar]
- Podolsky D. K., Lobb R., King N., Benjamin C. D., Pepinsky B., Sehgal P., deBeaumont M. Attenuation of colitis in the cotton-top tamarin by anti-alpha 4 integrin monoclonal antibody. J Clin Invest. 1993 Jul;92(1):372–380. doi: 10.1172/JCI116575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Ham R. G., Karmiol S. Expression of adhesion molecules in cultured human pulmonary microvascular endothelial cells. Microvasc Res. 1995 Nov;50(3):360–372. doi: 10.1006/mvre.1995.1064. [DOI] [PubMed] [Google Scholar]
- Stotland B. R., Lichtenstein G. R. Newer treatments for inflammatory bowel disease. Prim Care. 1996 Sep;23(3):577–608. doi: 10.1016/s0095-4543(05)70349-3. [DOI] [PubMed] [Google Scholar]
- Tailor A., Flower R. J., Perretti M. Dexamethasone inhibits leukocyte emigration in rat mesenteric post-capillary venules: an intravital microscopy study. J Leukoc Biol. 1997 Sep;62(3):301–308. doi: 10.1002/jlb.62.3.301. [DOI] [PubMed] [Google Scholar]
- Takei F. Inhibition of mixed lymphocyte response by a rat monoclonal antibody to a novel murine lymphocyte activation antigen (MALA-2). J Immunol. 1985 Mar;134(3):1403–1407. [PubMed] [Google Scholar]
- Travis S. P., Jewell D. P. Salicylates for inflammatory bowel disease. Baillieres Clin Gastroenterol. 1994 Jun;8(2):203–231. doi: 10.1016/0950-3528(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Zijlstra F. J., Garrelds I. M., van Dijk A. P., Wilson J. H. Experimental colitis in mice: effects of olsalazine on eicosanoid production in colonic tissue. Agents Actions. 1992;Spec No:C76–C78. [PubMed] [Google Scholar]