Abstract
Background—Polyethylene glycol (PEG) 3350 is a non-absorbable, non-metabolised osmotic agent used in lavage solutions for gut cleansing. Aims—To compare the efficacy of PEG and lactulose in chronic constipation. Methods—A total of 115 patients with chronic constipation entered a multicentre, randomised, comparative trial. They initially received two sachets containing either PEG (13 g/sachet) or lactulose (10 g/sachet) and were given an option to change the dose to one or three sachets/day, depending on response. Results—Ninety nine patients completed the trial. After four weeks, patients in the PEG group (n=50) had a higher number of stools and a lower median daily score for straining at stool than patients in the lactulose group (n=49). Overall improvement was greater in the PEG group. Clinical tolerance was similar in the two groups, but flatus was less frequently reported in the PEG group. The mean number of liquid stools was higher in the PEG group but the difference was significant only for the first two weeks. There were no serious adverse events and no significant change in laboratory tests in either group. At the end of the study, the number of sachets used by the patients was 1.6 (0.7)/day in the PEG group and 2.1 (0.7)/day in the lactulose group. Sixty one patients completed a further two months open study of one to three sachets PEG daily; there was no loss of efficacy and no serious toxicity. Conclusion—Low dose PEG 3350 was more effective than lactulose and better tolerated.
Keywords: constipation; polyethylene glycol; lactulose; cathartics; randomised trial
Full Text
The Full Text of this article is available as a PDF (122.7 KB).
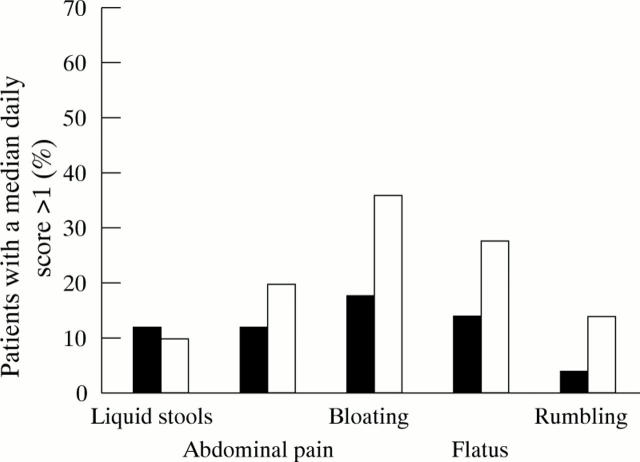
Figure 1 .
Clinical tolerance of PEG (black bars) and lactulose (white bars). The percentage of patients with a median daily score greater than 1 was significantly lower in the PEG group for bloating (p<0.05) and a trend was found for abdominal pain, flatus, and rumbling.
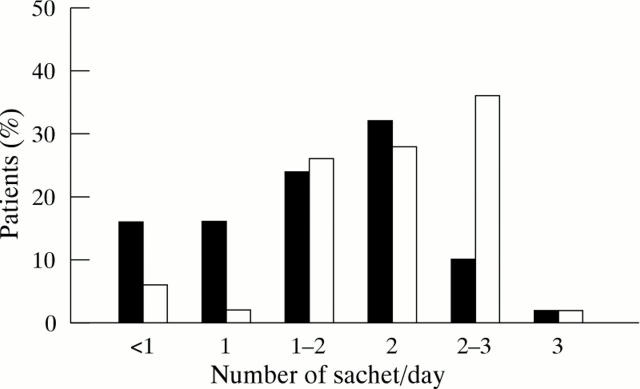
Figure 2 .
Percentage of patients taking the different dosages of PEG (black bars) and lactulose (white bars) during the last two weeks of the study.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andorsky R. I., Goldner F. Colonic lavage solution (polyethylene glycol electrolyte lavage solution) as a treatment for chronic constipation: a double-blind, placebo-controlled study. Am J Gastroenterol. 1990 Mar;85(3):261–265. [PubMed] [Google Scholar]
- Badiali D., Corazziari E., Habib F. I., Tomei E., Bausano G., Magrini P., Anzini F., Torsoli A. Effect of wheat bran in treatment of chronic nonorganic constipation. A double-blind controlled trial. Dig Dis Sci. 1995 Feb;40(2):349–356. doi: 10.1007/BF02065421. [DOI] [PubMed] [Google Scholar]
- Berry M. A., DiPalma J. A. Review article: orthograde gut lavage for colonoscopy. Aliment Pharmacol Ther. 1994 Aug;8(4):391–395. doi: 10.1111/j.1365-2036.1994.tb00305.x. [DOI] [PubMed] [Google Scholar]
- Brady C. E., 3rd, DiPalma J. A., Morawski S. G., Santa Ana C. A., Fordtran J. S. Urinary excretion of polyethylene glycol 3350 and sulfate after gut lavage with a polyethylene glycol electrolyte lavage solution. Gastroenterology. 1986 Jun;90(6):1914–1918. doi: 10.1016/0016-5085(86)90261-1. [DOI] [PubMed] [Google Scholar]
- Corazziari E., Badiali D., Habib F. I., Reboa G., Pitto G., Mazzacca G., Sabbatini F., Galeazzi R., Cilluffo T., Vantini I. Small volume isosmotic polyethylene glycol electrolyte balanced solution (PMF-100) in treatment of chronic nonorganic constipation. Dig Dis Sci. 1996 Aug;41(8):1636–1642. doi: 10.1007/BF02087913. [DOI] [PubMed] [Google Scholar]
- Florent C., Flourie B., Leblond A., Rautureau M., Bernier J. J., Rambaud J. C. Influence of chronic lactulose ingestion on the colonic metabolism of lactulose in man (an in vivo study). J Clin Invest. 1985 Feb;75(2):608–613. doi: 10.1172/JCI111738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis C. Y., Whorwell P. J. Bran and irritable bowel syndrome: time for reappraisal. Lancet. 1994 Jul 2;344(8914):39–40. doi: 10.1016/s0140-6736(94)91055-3. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Moser S. E., Estes M. K. The effect of bran on bowel function in constipation. Am J Gastroenterol. 1982 Sep;77(9):599–603. [PubMed] [Google Scholar]
- Hammer H. F., Santa Ana C. A., Schiller L. R., Fordtran J. S. Studies of osmotic diarrhea induced in normal subjects by ingestion of polyethylene glycol and lactulose. J Clin Invest. 1989 Oct;84(4):1056–1062. doi: 10.1172/JCI114267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauser A. G., Mühldorfer B. E., Voderholzer W. A., Wenzel G., Müller-Lissner S. A. Polyethylene glycol 4000 for slow transit constipation. Z Gastroenterol. 1995 Jan;33(1):5–8. [PubMed] [Google Scholar]
- Lederle F. A., Busch D. L., Mattox K. M., West M. J., Aske D. M. Cost-effective treatment of constipation in the elderly: a randomized double-blind comparison of sorbitol and lactulose. Am J Med. 1990 Nov;89(5):597–601. doi: 10.1016/0002-9343(90)90177-f. [DOI] [PubMed] [Google Scholar]
- Passmore A. P., Wilson-Davies K., Stoker C., Scott M. E. Chronic constipation in long stay elderly patients: a comparison of lactulose and a senna-fibre combination. BMJ. 1993 Sep 25;307(6907):769–771. doi: 10.1136/bmj.307.6907.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puxty J. A., Fox R. A. Golytely: a new approach to faecal impaction in old age. Age Ageing. 1986 May;15(3):182–184. doi: 10.1093/ageing/15.3.182. [DOI] [PubMed] [Google Scholar]
- SMYTH H. F., Jr, CARPENTER C. P., WEIL C. S. The chronic oral toxicology of the polyethylene glycols. J Am Pharm Assoc Am Pharm Assoc. 1955 Jan;44(1):27–30. doi: 10.1002/jps.3030440111. [DOI] [PubMed] [Google Scholar]
- Sanders J. F. Lactulose syrup assessed in a double-blind study of elderly constipated patients. J Am Geriatr Soc. 1978 May;26(5):236–239. doi: 10.1111/j.1532-5415.1978.tb01967.x. [DOI] [PubMed] [Google Scholar]
- Thompson W. G. Laxatives: clinical pharmacology and rational use. Drugs. 1980 Jan;19(1):49–58. doi: 10.2165/00003495-198019010-00004. [DOI] [PubMed] [Google Scholar]
- Tolia V., Lin C. H., Elitsur Y. A prospective randomized study with mineral oil and oral lavage solution for treatment of faecal impaction in children. Aliment Pharmacol Ther. 1993 Oct;7(5):523–529. doi: 10.1111/j.1365-2036.1993.tb00128.x. [DOI] [PubMed] [Google Scholar]