Abstract
Background—Reactive oxygen and nitrogen derived species produced by activated neutrophils have been implicated in the damage of mucosal proteins including the inhibition of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the active inflammatory lesion in patients with inflammatory bowel disease (IBD). This study investigated the efficacy of currently used IBD therapeutics to prevent injury mediated by reactive oxygen and nitrogen derived species. Methods—GAPDH activity of human colon epithelial cells was used as a sensitive indicator of injury produced by reactive oxygen and nitrogen derived species. HCT116 cells (106/ml phosphate buffered saline; 37°C) were incubated in the presence of 5-aminosalicylic acid (5-ASA), 6-mercaptopurine, methylprednisolone, or metronidazole before exposure to H2O2, HOCl, or NO in vitro. HCT116 cell GAPDH enzyme activity was determined by standard procedures. Cell free reactions between 5-ASA and HOCl were analysed by spectrophotometry and fluorimetry to characterise the mechanism of oxidant scavenging. Results—GAPDH activity of HCT116 cells was inhibited by the oxidants tested: the concentration that produced 50% inhibition (IC50) was 44.5 (2.1) µM for HOCl, 379.8 (21.3) µM for H2O2, and 685.8 (103.8) µM for NO (means (SEM)). 5-ASA was the only therapeutic compound tested to show efficacy (p<0.05) against HOCl mediated inhibition of enzyme activity; however, it was ineffective against H2O2 and NO mediated inhibition of GAPDH. Methylprednisolone, metronidazole, and the thiol-containing 6-mercaptopurine were ineffective against all oxidants. Studies at ratios of HOCl:5-ASA achievable in the mucosa showed direct scavenging to be the mechanism of protection of GAPDH activity. Mixing 5-ASA and HOCl before addition to the cells resulted in significantly greater protection of GAPDH activity than when HOCl was added to cells preincubated with 5-ASA. The addition of 5-ASA after HOCl exposure did not restore GAPDH activity. Conclusions—Therapies based on 5-ASA may play a direct role in scavenging the potent neutrophil oxidant HOCl, thereby protecting mucosal GAPDH from oxidative inhibition. These findings suggest that strategies for the further development of new HOCl scavenging compounds may be useful in the treatment of IBD.
Keywords: 5-aminosalicylic acid; 6-mercaptopurine; prednisolone; metronidazole; oxidants; glyceraldehyde-3-phosphate dehydrogenase
Full Text
The Full Text of this article is available as a PDF (153.8 KB).
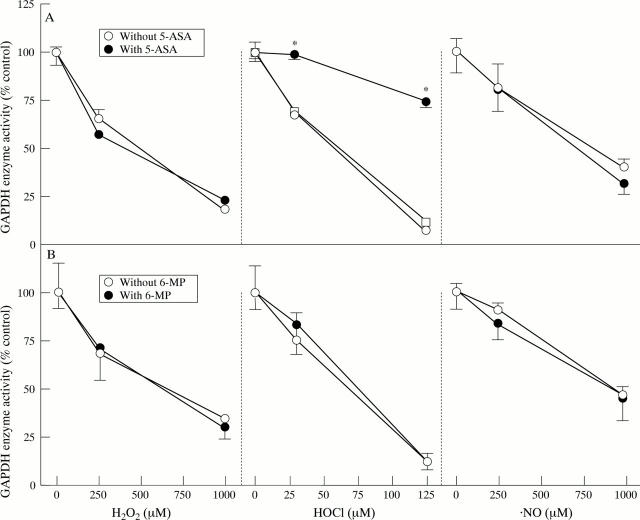
Figure 1 .
Efficacy of 5-aminosalicylic acid (5-ASA) and 6-mercaptopurine (6-MP) against oxidant mediated inhibition of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity. HCT116 cells were exposed to H2O2, HOCl, or NO after preincubation (A) in the absence or presence of 5-ASA or incubated with 5-ASA after exposure to HOCl or (B) in the absence or presence of 6-mercaptopurine. Oxidant and therapeutic concentrations were as described in the Materials and methods section. Data represent mean (SEM) (n = 3). *p<0.05 compared with no 5-ASA.
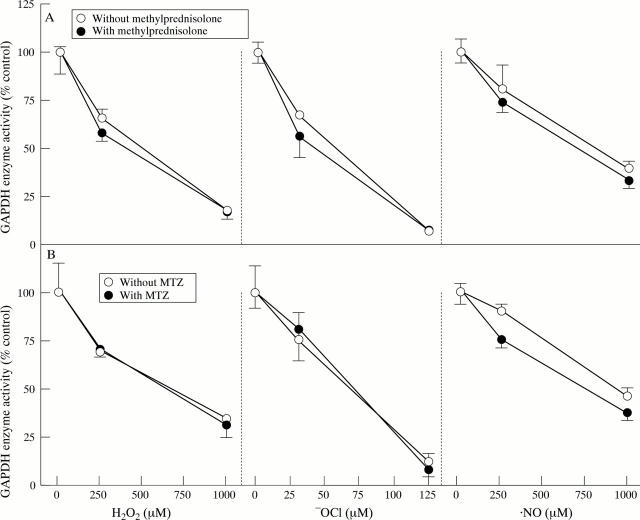
Figure 2 .
Efficacy of methylprednisolone and metronidazole against oxidant mediated inhibition of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity. HCT116 cells were exposed to H2O2, HOCl, or NO after preincubation (A) in the absence or presence of methylprednisolone or (B) in the absence or presence of metronidazole (MTZ). Oxidant and therapeutic concentrations were as described in the Materials and methods section. Data represent mean (SEM) (n = 3).
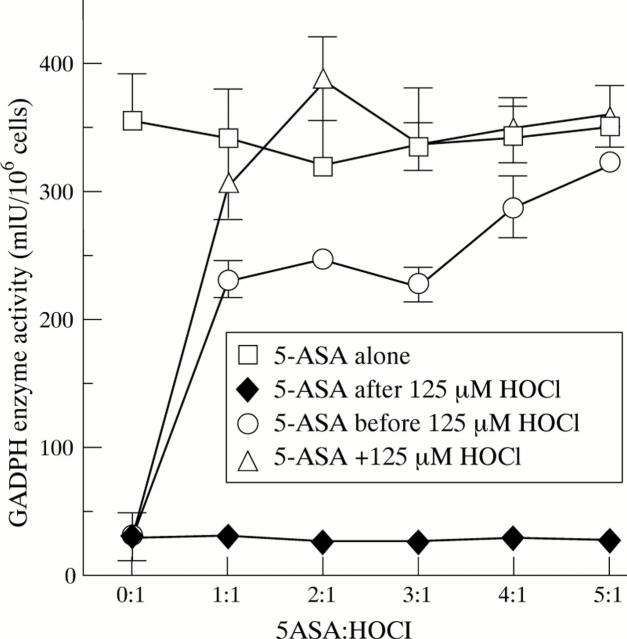
Figure 3 .
Dose-dependence of 5-aminosalicylic acid (5-ASA) prevention of the inhibition of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity mediated by HOCl. HCT116 cells were exposed to increasing ratios of 5-ASA:HOCl according to the following protocol: 5-ASA alone, 5-ASA after exposure to 125 µM HOCl, 5-ASA before exposure to 125 µM HOCl, or the spent reaction resulting from the premixing of 5-ASA with 125 µM HOCl. Data represent mean (SEM) (n = 3).
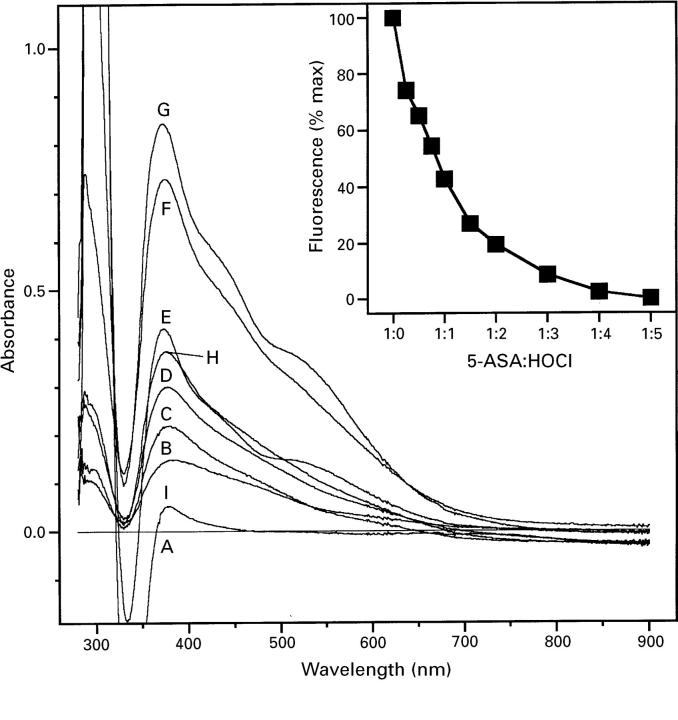
Figure 4 .
Reaction of 5-aminosalicylic acid (5-ASA) with HOCl. Increasing concentrations of HOCl were allowed to react with 5-ASA as described in the Materials and methods section and differential absorbance spectrums determined. The reference cuvette contained 250 µM 5-ASA in HBSS, and the sample cuvette increasing ratios of 5-ASA:HOCl; 37°C. Absorbance traces of 5-ASA:HOCl were as follows: A, 1:0; B, 1:0.25; C, 1:0.5; D, 1:0.75; E, 1:1; F, 1:2; G, 1:3; H, 1:4; I, 1:5. Insert, 5-ASA fluorescence.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnfelt-Rønne I., Nielsen O. H., Christensen A., Langholz E., Binder V., Riis P. Clinical evidence supporting the radical scavenger mechanism of 5-aminosalicylic acid. Gastroenterology. 1990 May;98(5 Pt 1):1162–1169. doi: 10.1016/0016-5085(90)90329-y. [DOI] [PubMed] [Google Scholar]
- Aruoma O. I., Wasil M., Halliwell B., Hoey B. M., Butler J. The scavenging of oxidants by sulphasalazine and its metabolites. A possible contribution to their anti-inflammatory effects? Biochem Pharmacol. 1987 Nov 1;36(21):3739–3742. doi: 10.1016/0006-2952(87)90028-1. [DOI] [PubMed] [Google Scholar]
- Babbs C. F. Oxygen radicals in ulcerative colitis. Free Radic Biol Med. 1992;13(2):169–181. doi: 10.1016/0891-5849(92)90079-v. [DOI] [PubMed] [Google Scholar]
- Blackburn A. C., Doe W. F., Buffinton G. D. Salicylate hydroxylation as an indicator of hydroxyl radical generation in dextran sulfate-induced colitis. Free Radic Biol Med. 1998 Aug;25(3):305–313. doi: 10.1016/s0891-5849(98)00068-9. [DOI] [PubMed] [Google Scholar]
- Buffinton G. D., Doe W. F. Altered ascorbic acid status in the mucosa from inflammatory bowel disease patients. Free Radic Res. 1995 Feb;22(2):131–143. doi: 10.3109/10715769509147535. [DOI] [PubMed] [Google Scholar]
- Buffinton G. D., Doe W. F. Depleted mucosal antioxidant defences in inflammatory bowel disease. Free Radic Biol Med. 1995 Dec;19(6):911–918. doi: 10.1016/0891-5849(95)94362-h. [DOI] [PubMed] [Google Scholar]
- Buffinton G., Cadenas E. Reduction of ferrylmyoglobin to metmyoglobin by quinonoid compounds. Chem Biol Interact. 1988;66(3-4):233–250. doi: 10.1016/0009-2797(88)90074-9. [DOI] [PubMed] [Google Scholar]
- Buffinton G., Mira D., Galaris D., Hochstein P., Cadenas E. Reduction of ferryl- and metmyoglobin to ferrous myoglobin by menadione-glutathione conjugate. Spectrophotometric studies under aerobic and anaerobic conditions. Chem Biol Interact. 1988;66(3-4):205–222. doi: 10.1016/0009-2797(88)90072-5. [DOI] [PubMed] [Google Scholar]
- Campieri M., Lanfranchi G. A., Bertoni F., Brignola C., Bazzocchi G., Minguzzi M. R., Labò G. A double-blind clinical trial to compare the effects of 4-aminosalicylic acid to 5-aminosalicylic acid in topical treatment of ulcerative colitis. Digestion. 1984;29(4):204–208. doi: 10.1159/000199034. [DOI] [PubMed] [Google Scholar]
- Carlin G., Djursäter R., Smedegård G., Gerdin B. Effect of anti-inflammatory drugs on xanthine oxidase and xanthine oxidase induced depolymerization of hyaluronic acid. Agents Actions. 1985 Jul;16(5):377–384. doi: 10.1007/BF01982876. [DOI] [PubMed] [Google Scholar]
- Dull B. J., Salata K., Van Langenhove A., Goldman P. 5-Aminosalicylate: oxidation by activated leukocytes and protection of cultured cells from oxidative damage. Biochem Pharmacol. 1987 Aug 1;36(15):2467–2472. doi: 10.1016/0006-2952(87)90518-1. [DOI] [PubMed] [Google Scholar]
- Emerit J., Pelletier S., Likforman J., Pasquier C., Thuillier A. Phase II trial of copper zinc superoxide dismutase (CuZn SOD) in the treatment of Crohn's disease. Free Radic Res Commun. 1991;12-13 Pt 2:563–569. doi: 10.3109/10715769109145831. [DOI] [PubMed] [Google Scholar]
- Galaris D., Cadenas E., Hochstein P. Glutathione-dependent reduction of peroxides during ferryl- and met-myoglobin interconversion: a potential protective mechanism in muscle. Free Radic Biol Med. 1989;6(5):473–478. doi: 10.1016/0891-5849(89)90039-7. [DOI] [PubMed] [Google Scholar]
- Galaris D., Cadenas E., Hochstein P. Redox cycling of myoglobin and ascorbate: a potential protective mechanism against oxidative reperfusion injury in muscle. Arch Biochem Biophys. 1989 Sep;273(2):497–504. doi: 10.1016/0003-9861(89)90509-2. [DOI] [PubMed] [Google Scholar]
- Grimm M. C., Elsbury S. K., Pavli P., Doe W. F. Interleukin 8: cells of origin in inflammatory bowel disease. Gut. 1996 Jan;38(1):90–98. doi: 10.1136/gut.38.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham M. B., Gaginella T. S., von Ritter C., Tamai H., Be R. M., Granger D. N. Effects of neutrophil-derived oxidants on intestinal permeability, electrolyte transport, and epithelial cell viability. Inflammation. 1990 Oct;14(5):531–542. doi: 10.1007/BF00914274. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Granger D. N. 5-Aminosalicylic acid concentration in mucosal interstitium of cat small and large intestine. Dig Dis Sci. 1989 Apr;34(4):573–578. doi: 10.1007/BF01536335. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., MacDermott R. P., Deitch E. A. Oxidant defense mechanisms in the human colon. Inflammation. 1990 Dec;14(6):669–680. doi: 10.1007/BF00916370. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Miles A. M. Effects of aminosalicylates and immunosuppressive agents on nitric oxide-dependent N-nitrosation reactions. Biochem Pharmacol. 1994 May 18;47(10):1897–1902. doi: 10.1016/0006-2952(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Jensen J., Cornett C., Olsen C. E., Bondesen S., Christensen J., Christensen L. A., Tjørnelund J., Hansen S. H. Identification of oxidation products of 5-aminosalicylic acid in faeces and the study of their formation in vitro. Biochem Pharmacol. 1993 Mar 24;45(6):1201–1209. doi: 10.1016/0006-2952(93)90271-w. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A., Morgan G., Sedghi S., Gordon J. H., Doria M. Role of reactive oxygen metabolites in experimental colitis. Gut. 1990 Jul;31(7):786–790. doi: 10.1136/gut.31.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffafian I., Brown R. C., Hallett M. B. The production of an amine-modified derivative of 5-aminosalicylic acid by activated neutrophils. Roles for myeloperoxidase and chloride ions. Biochem Pharmacol. 1991 Oct 24;42(10):1869–1874. doi: 10.1016/0006-2952(91)90583-q. [DOI] [PubMed] [Google Scholar]
- McKenzie S. J., Baker M. S., Buffinton G. D., Doe W. F. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest. 1996 Jul 1;98(1):136–141. doi: 10.1172/JCI118757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyachi Y., Yoshioka A., Imamura S., Niwa Y. Effect of sulphasalazine and its metabolites on the generation of reactive oxygen species. Gut. 1987 Feb;28(2):190–195. doi: 10.1136/gut.28.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa Y., Somiya K., Michelson A. M., Puget K. Effect of liposomal-encapsulated superoxide dismutase on active oxygen-related human disorders. A preliminary study. Free Radic Res Commun. 1985;1(2):137–153. doi: 10.3109/10715768509056547. [DOI] [PubMed] [Google Scholar]
- Sartor R. B. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994 Feb;106(2):533–539. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- Tamai H., Kachur J. F., Grisham M. B., Gaginella T. S. Scavenging effect of 5-aminosalicylic acid on neutrophil-derived oxidants. Possible contribution to the mechanism of action in inflammatory bowel disease. 1991 Mar 15-Apr 1Biochem Pharmacol. 41(6-7):1001–1006. doi: 10.1016/0006-2952(91)90207-l. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Hallett M. B. The reaction of 5-amino-salicylic acid with hypochlorite. Implications for its mode of action in inflammatory bowel disease. Biochem Pharmacol. 1989 Jan 1;38(1):149–154. doi: 10.1016/0006-2952(89)90161-5. [DOI] [PubMed] [Google Scholar]
- Yamada T., Volkmer C., Grisham M. B. The effects of sulfasalazine metabolites on hemoglobin-catalyzed lipid peroxidation. Free Radic Biol Med. 1991;10(1):41–49. doi: 10.1016/0891-5849(91)90020-4. [DOI] [PubMed] [Google Scholar]
- von Ritter C., Grisham M. B., Granger D. N. Sulfasalazine metabolites and dapsone attenuate formyl-methionyl-leucyl-phenylalanine-induced mucosal injury in rat ileum. Gastroenterology. 1989 Mar;96(3):811–816. [PubMed] [Google Scholar]
- von Ritter C., Grisham M. B., Hollwarth M., Inauen W., Granger D. N. Neutrophil-derived oxidants mediate formyl-methionyl-leucyl-phenylalanine-induced increases in mucosal permeability in rats. Gastroenterology. 1989 Sep;97(3):778–780. doi: 10.1016/0016-5085(89)90654-9. [DOI] [PubMed] [Google Scholar]