Abstract
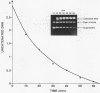
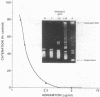
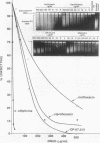
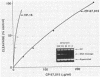
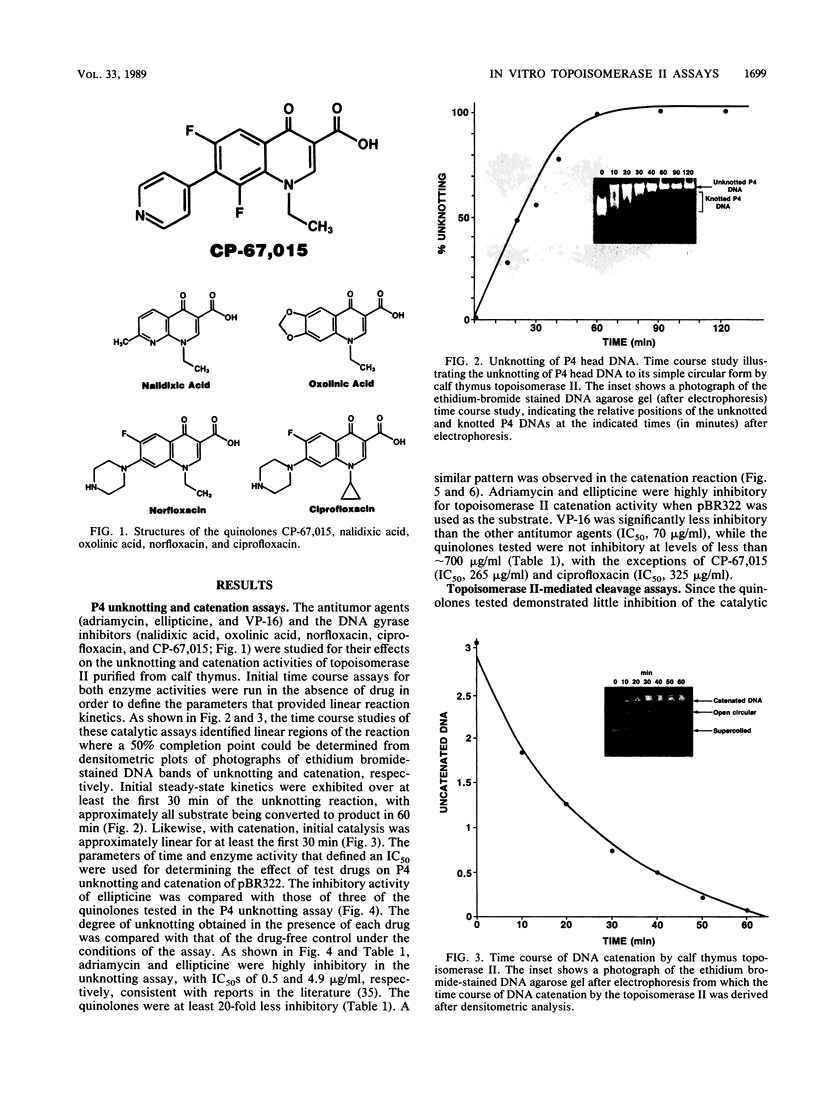
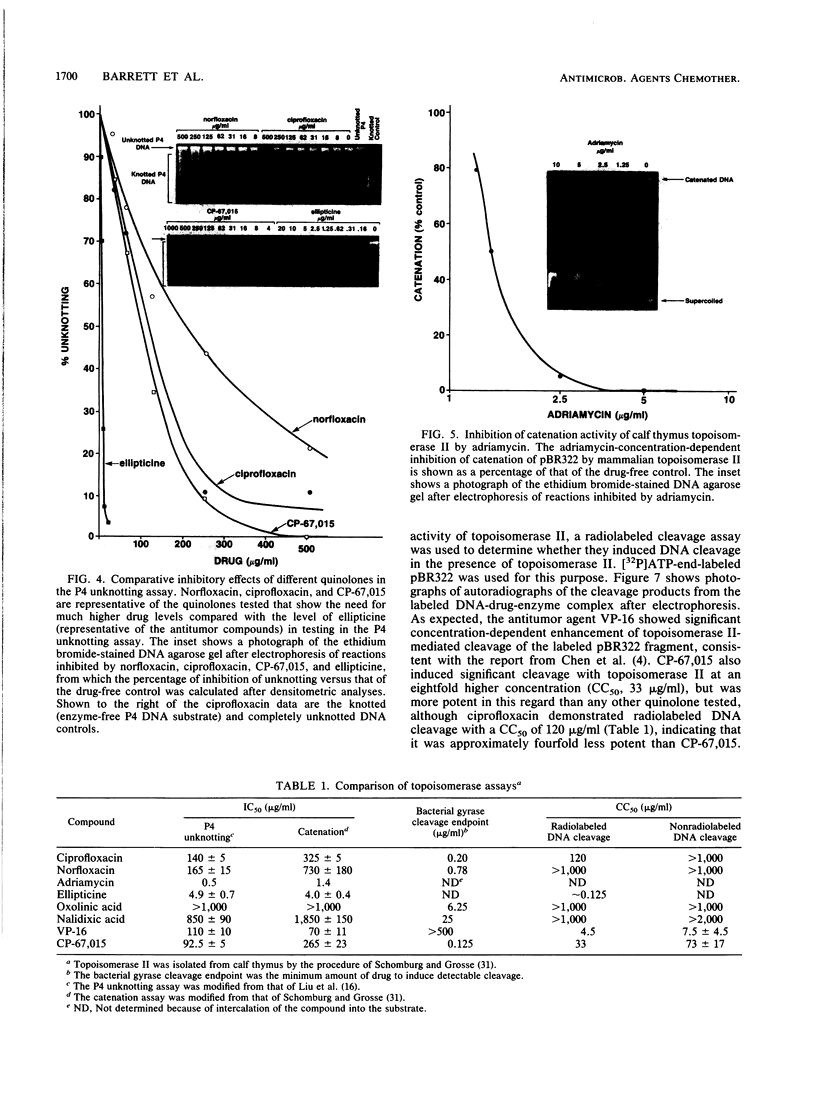
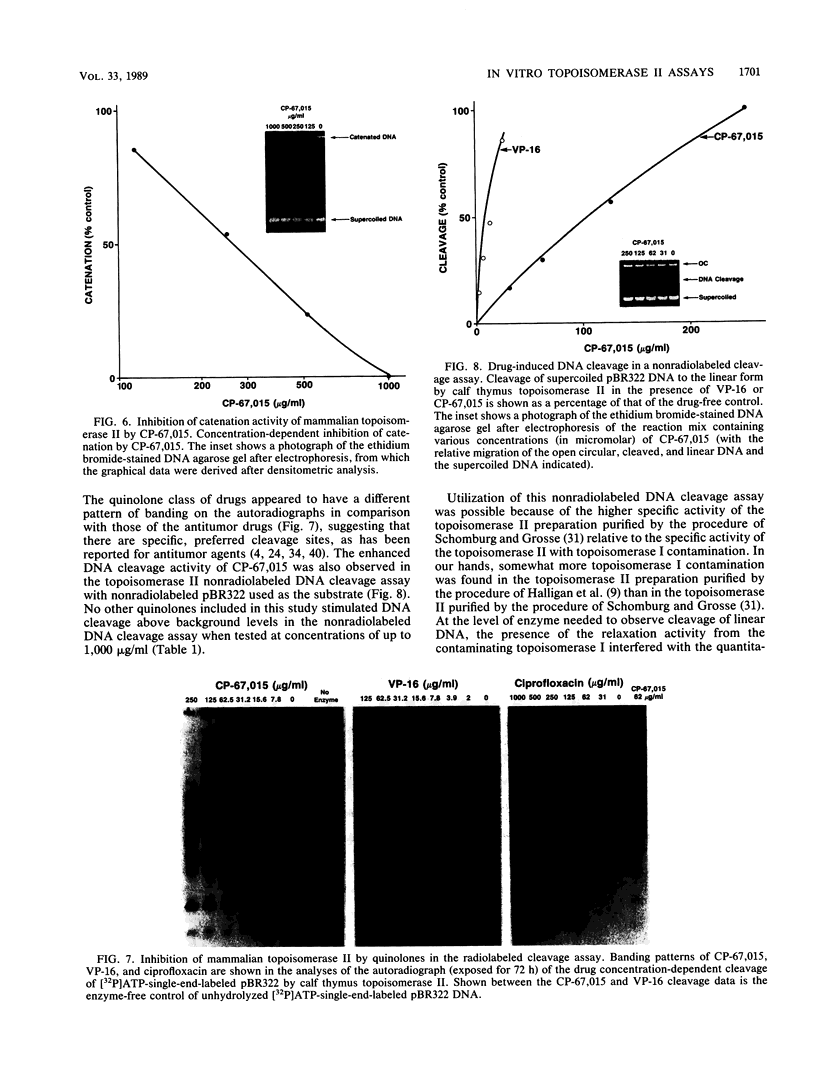
Several quinolones and antitumor compounds were tested as inhibitors of purified calf thymus topoisomerase II in unknotting, catenation, radiolabeled DNA cleavage, and quantitative nonradiolabeled cleavage assays. The antitumor agents VP-16 (demethylepipodophyllotoxin ethylio-beta-D-glucoside) and ellipticine demonstrated drug-enhanced topoisomerase II DNA cleavage (the concentration of drug that induced 50% of the maximal DNA cleavage in the test system [CC50]) at levels of less than or equal to 5 micrograms/ml. Nalidixic acid, norfloxacin, and oxolinic acid did not induce significant topoisomerase II DNA cleavage, whereas ciprofloxacin did induce some cleavage above background levels. CP-67,015, a new 6,8-difluoro-7-pyridyl 4-quinolone which possesses potent antibacterial activity, inhibited bacterial DNA gyrase at 0.125 micrograms/ml in a nonradioactive DNA cleavage assay. Unlike other quinolones characterized to date, CP-67,015 was shown to strongly enhance topoisomerase II-induced radiolabeled DNA cleavage with a CC50 of 33 micrograms/ml and demonstrated cleavage in a nonradiolabeled DNA cleavage assay with a CC50 of 73 micrograms/ml. The topoisomerase II-mediated cleavage of DNA by CP-67,015 is consistent with its reported clastogenic effect on DNA in cell culture and its positive mutagenic response in mouse lymphoma cells. In vitro topoisomerase II catalytic and cleavage assays are useful for gaining preliminary information concerning the possible interaction(s) of some quinolones with eucaryotic topoisomerase II which may relate directly to their safety (mutagenicity, clastogenicity, or both) in human and veterinary medicinal usage.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auclair C. Multimodal action of antitumor agents on DNA: the ellipticine series. Arch Biochem Biophys. 1987 Nov 15;259(1):1–14. doi: 10.1016/0003-9861(87)90463-2. [DOI] [PubMed] [Google Scholar]
- Chen G. L., Yang L., Rowe T. C., Halligan B. D., Tewey K. M., Liu L. F. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Nov 10;259(21):13560–13566. [PubMed] [Google Scholar]
- Christ W., Lehnert T., Ulbrich B. Specific toxicologic aspects of the quinolones. Rev Infect Dis. 1988 Jan-Feb;10 (Suppl 1):S141–S146. doi: 10.1093/clinids/10.supplement_1.s141. [DOI] [PubMed] [Google Scholar]
- Drlica K., Franco R. J. Inhibitors of DNA topoisomerases. Biochemistry. 1988 Apr 5;27(7):2253–2259. doi: 10.1021/bi00407a001. [DOI] [PubMed] [Google Scholar]
- Forsgren A., Bredberg A., Pardee A. B., Schlossman S. F., Tedder T. F. Effects of ciprofloxacin on eucaryotic pyrimidine nucleotide biosynthesis and cell growth. Antimicrob Agents Chemother. 1987 May;31(5):774–779. doi: 10.1128/aac.31.5.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halligan B. D., Edwards K. A., Liu L. F. Purification and characterization of a type II DNA topoisomerase from bovine calf thymus. J Biol Chem. 1985 Feb 25;260(4):2475–2482. [PubMed] [Google Scholar]
- Holden H. E., Barett J. F., Huntington C. M., Muehlbauer P. A., Wahrenburg M. G. Genetic profile of a nalidixic acid analog: a model for the mechanism of sister chromatid exchange induction. Environ Mol Mutagen. 1989;13(3):238–252. doi: 10.1002/em.2850130308. [DOI] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S. Mode of action of the quinolone antimicrobial agents. Rev Infect Dis. 1988 Jan-Feb;10 (Suppl 1):S14–S21. doi: 10.1093/clinids/10.supplement_1.s14. [DOI] [PubMed] [Google Scholar]
- Hussy P., Maass G., Tümmler B., Grosse F., Schomburg U. Effect of 4-quinolones and novobiocin on calf thymus DNA polymerase alpha primase complex, topoisomerases I and II, and growth of mammalian lymphoblasts. Antimicrob Agents Chemother. 1986 Jun;29(6):1073–1078. doi: 10.1128/aac.29.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley D. M. DNA supercoiling. Biochem Soc Trans. 1986 Apr;14(2):489–493. doi: 10.1042/bst0140489. [DOI] [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerases--enzymes that catalyse the breaking and rejoining of DNA. CRC Crit Rev Biochem. 1983;15(1):1–24. doi: 10.3109/10409238309102799. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Davis J. L., Calendar R. Novel topologically knotted DNA from bacteriophage P4 capsids: studies with DNA topoisomerases. Nucleic Acids Res. 1981 Aug 25;9(16):3979–3989. doi: 10.1093/nar/9.16.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F., Liu C. C., Alberts B. M. Type II DNA topoisomerases: enzymes that can unknot a topologically knotted DNA molecule via a reversible double-strand break. Cell. 1980 Mar;19(3):697–707. doi: 10.1016/s0092-8674(80)80046-8. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Rowe T. C., Yang L., Tewey K. M., Chen G. L. Cleavage of DNA by mammalian DNA topoisomerase II. J Biol Chem. 1983 Dec 25;258(24):15365–15370. [PubMed] [Google Scholar]
- Lock R. B., Ross W. E. DNA topoisomerases in cancer therapy. Anticancer Drug Des. 1987 Oct;2(2):151–164. [PubMed] [Google Scholar]
- Maxwell A., Gellert M. Mechanistic aspects of DNA topoisomerases. Adv Protein Chem. 1986;38:69–107. doi: 10.1016/s0065-3233(08)60526-4. [DOI] [PubMed] [Google Scholar]
- Minford J., Pommier Y., Filipski J., Kohn K. W., Kerrigan D., Mattern M., Michaels S., Schwartz R., Zwelling L. A. Isolation of intercalator-dependent protein-linked DNA strand cleavage activity from cell nuclei and identification as topoisomerase II. Biochemistry. 1986 Jan 14;25(1):9–16. doi: 10.1021/bi00349a002. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K., O'Dea M. H., Gellert M. DNA gyrase: subunit structure and ATPase activity of the purified enzyme. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5960–5963. doi: 10.1073/pnas.75.12.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. M., Tewey K. M., Liu L. F. Mechanism of antitumor drug action: poisoning of mammalian DNA topoisomerase II on DNA by 4'-(9-acridinylamino)-methanesulfon-m-anisidide. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1361–1365. doi: 10.1073/pnas.81.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomori Y., Yasue T., Aoyama H., Hirai K., Suzue S., Yokota T. Effects of fleroxacin on HeLa cell functions and topoisomerase II. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):91–97. doi: 10.1093/jac/22.supplement_d.91. [DOI] [PubMed] [Google Scholar]
- Phillips I. Bacterial mutagenicity and the 4-quinolones. J Antimicrob Chemother. 1987 Dec;20(6):771–773. doi: 10.1093/jac/20.6.771. [DOI] [PubMed] [Google Scholar]
- Pommier Y., Mattern M. R., Schwartz R. E., Zwelling L. A. Absence of swiveling at sites of intercalator-induced protein-associated deoxyribonucleic acid strand breaks in mammalian cell nucleoids. Biochemistry. 1984 Jun 19;23(13):2922–2927. doi: 10.1021/bi00308a011. [DOI] [PubMed] [Google Scholar]
- Pommier Y., Mattern M. R., Schwartz R. E., Zwelling L. A., Kohn K. W. Changes in deoxyribonucleic acid linking number due to treatment of mammalian cells with the intercalating agent 4'-(9-acridinylamino)methanesulfon-m-anisidide. Biochemistry. 1984 Jun 19;23(13):2927–2932. doi: 10.1021/bi00308a012. [DOI] [PubMed] [Google Scholar]
- Pommier Y., Minford J. K., Schwartz R. E., Zwelling L. A., Kohn K. W. Effects of the DNA intercalators 4'-(9-acridinylamino)methanesulfon-m-anisidide and 2-methyl-9-hydroxyellipticinium on topoisomerase II mediated DNA strand cleavage and strand passage. Biochemistry. 1985 Nov 5;24(23):6410–6416. doi: 10.1021/bi00344a015. [DOI] [PubMed] [Google Scholar]
- Riou G., Douc-Rasy S., Kayser A. Inhibitors of trypanosome topoisomerases. Biochem Soc Trans. 1986 Apr;14(2):496–499. doi: 10.1042/bst0140496. [DOI] [PubMed] [Google Scholar]
- Schomburg U., Grosse F. Purification and characterization of DNA topoisomerase II from calf thymus associated with polypeptides of 175 and 150 kDa. Eur J Biochem. 1986 Nov 3;160(3):451–457. doi: 10.1111/j.1432-1033.1986.tb10061.x. [DOI] [PubMed] [Google Scholar]
- Shen L. L., Pernet A. G. Mechanism of inhibition of DNA gyrase by analogues of nalidixic acid: the target of the drugs is DNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):307–311. doi: 10.1073/pnas.82.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewey K. M., Chen G. L., Nelson E. M., Liu L. F. Intercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Jul 25;259(14):9182–9187. [PubMed] [Google Scholar]
- Tewey K. M., Rowe T. C., Yang L., Halligan B. D., Liu L. F. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984 Oct 26;226(4673):466–468. doi: 10.1126/science.6093249. [DOI] [PubMed] [Google Scholar]
- Tornaletti S., Pedrini A. M. Studies on the interaction of 4-quinolones with DNA by DNA unwinding experiments. Biochim Biophys Acta. 1988 Mar 31;949(3):279–287. doi: 10.1016/0167-4781(88)90153-4. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Webster A., Gaya H. Quinolones in the treatment of serious infections. Rev Infect Dis. 1988 Jan-Feb;10 (Suppl 1):S225–S233. doi: 10.1093/clinids/10.supplement_1.s225. [DOI] [PubMed] [Google Scholar]
- Woynarowski J. M., Sigmund R. D., Beerman T. A. Topoisomerase-II-mediated lesions in nascent DNA: comparison of the effects of epipodophyllotoxin derivatives, VM-26 and VP-16, and 9-anilinoacridine derivatives, m-AMSA and o-AMSA. Biochim Biophys Acta. 1988 May 6;950(1):21–29. doi: 10.1016/0167-4781(88)90069-3. [DOI] [PubMed] [Google Scholar]
- Yang L., Rowe T. C., Nelson E. M., Liu L. F. In vivo mapping of DNA topoisomerase II-specific cleavage sites on SV40 chromatin. Cell. 1985 May;41(1):127–132. doi: 10.1016/0092-8674(85)90067-4. [DOI] [PubMed] [Google Scholar]