Abstract
BACKGROUND—Dietary fibre influences the turnover and differentiation of the colonic epithelium, but its effects on barrier function are unknown. AIMS—To determine whether altering the type and amount of fibre in the diet affects paracellular permeability of intestinal epithelium, and to identify the mechanisms of action. METHODS—Rats were fed isoenergetic low fibre diets with or without supplements of wheat bran (10%) or methylcellulose (10%), for four weeks. Paracellular permeability was determined by measurement of conductance and 51Cr-EDTA flux across tissue mounted in Ussing chambers. Faecal short chain fatty acid (SCFA) concentrations were assessed by gas chromatography, epithelial kinetics stathmokinetically, and mucosal brush border hydrolase activities spectrophotometrically. RESULTS—Body weight was similar across the dietary groups. Conductance and 51Cr-EDTA flux were approximately 25% higher in animals fed no fibre, compared with those fed wheat bran or methylcellulose in the distal colon, but not in the caecum or jejunum. Histologically, there was no evidence of epithelial injury or erosion associated with any diet. The fibres exerted different spectra of effects on luminal SCFA concentrations and pH, and on mucosal indexes, but both bulked the faeces, were trophic to the epithelium, and stimulated expression of a marker of epithelial differentiation. CONCLUSIONS—Both a fermentable and a non-fermentable fibre reduce paracellular permeability specifically in the distal colon, possibly by promoting epithelial cell differentiation. The mechanisms by which the two fibres exert their effects are likely to be different.
Keywords: colon; differentiation; epithelium; fibre; paracellular permeability; proliferation
Full Text
The Full Text of this article is available as a PDF (128.6 KB).
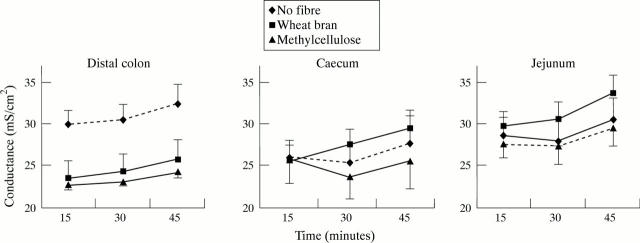
Figure 1 .
Effect of diet on transepithelial conductance in rat intestine. Data are expressed as mean (SEM) from 12 animals. ANOVA of area under the curve summary measures showed a significant difference across the dietary groups (p<0.001) for the distal colon only and, on multiple comparisons, wheat bran and methylcellulose groups were significantly different to the no fibre group (p<0.01).
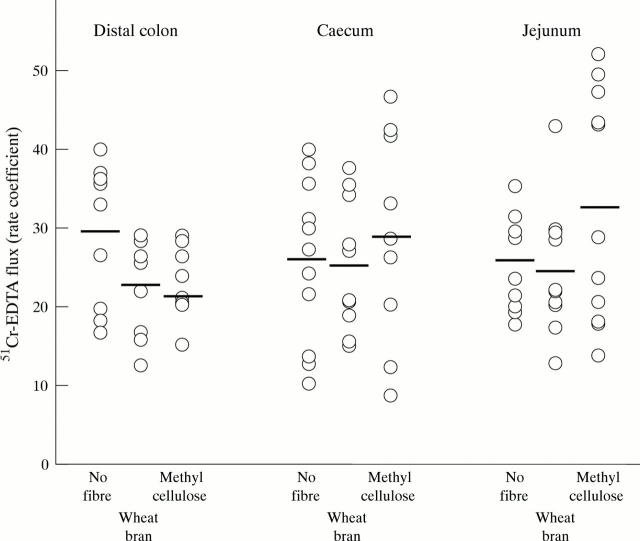
Figure 2 .
Effect of diet on the flux of 51Cr-EDTA across distal colon, caecum, and jejunum mounted in Ussing chambers. The diets contained no fibre, wheat bran, or methylcellulose. For the distal colon only, differences were statistically significant across the dietary groups (p=0.004; ANOVA). EDTA flux in the wheat bran and methylcellulose groups was significantly different to that in the no fibre group on multiple comparisons (p<0.05).
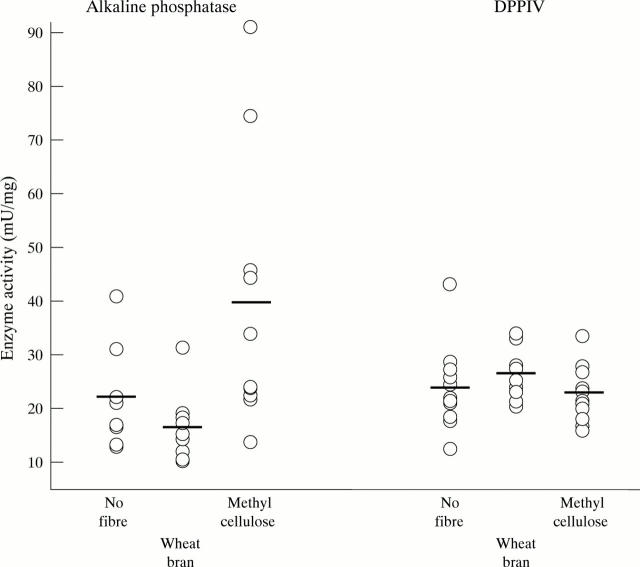
Figure 3 .
Effect of diet on brush border hydrolase activities of distal colonic mucosa. The diets contained no fibre, wheat bran, or methylcellulose. Alkaline phosphatase and dipeptidylpeptidase IV (DPPIV) activities differed significantly across the dietary groups (p<0.001 and p<0.05 respectively, ANOVA). Alkaline phosphatase activity in the methylcellulose group and DPPIV activity in the wheat bran group were significantly different to those in the other groups on multiple comparisons (p<0.05).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almer S., Franzén L., Olaison G., Smedh K., Ström M. Increased absorption of polyethylene glycol 600 deposited in the colon in active ulcerative colitis. Gut. 1993 Apr;34(4):509–513. doi: 10.1136/gut.34.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson M. D., Hong F., Emenaker N. J. Specific modulation of intestinal epithelial brush border enzyme expression by a phorbol ester. J Surg Res. 1995 Jul;59(1):121–126. doi: 10.1006/jsre.1995.1142. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burnham D. B. Epithelial cell production and mucosal morphology in colonic obstruction. Cell Tissue Res. 1983;230(1):185–196. doi: 10.1007/BF00216038. [DOI] [PubMed] [Google Scholar]
- D'Argenio G., Cosenza V., Delle Cave M., Iovino P., Delle Valle N., Lombardi G., Mazzacca G. Butyrate enemas in experimental colitis and protection against large bowel cancer in a rat model. Gastroenterology. 1996 Jun;110(6):1727–1734. doi: 10.1053/gast.1996.v110.pm8964397. [DOI] [PubMed] [Google Scholar]
- Dowling R. H., Riecken E. O., Laws J. W., Booth C. C. The intestinal response to high bulk feeding in the rat. Clin Sci. 1967 Feb;32(1):1–9. [PubMed] [Google Scholar]
- Folino M., McIntyre A., Young G. P. Dietary fibers differ in their effects on large bowel epithelial proliferation and fecal fermentation-dependent events in rats. J Nutr. 1995 Jun;125(6):1521–1528. doi: 10.1093/jn/125.6.1521. [DOI] [PubMed] [Google Scholar]
- Gibson P. R. Ulcerative colitis: an epithelial disease? Baillieres Clin Gastroenterol. 1997 Mar;11(1):17–33. doi: 10.1016/s0950-3528(97)90051-8. [DOI] [PubMed] [Google Scholar]
- Glotzer D. J., Glick M. E., Goldman H. Proctitis and colitis following diversion of the fecal stream. Gastroenterology. 1981 Mar;80(3):438–441. [PubMed] [Google Scholar]
- Halline A. G., Davidson N. O., Skarosi S. F., Sitrin M. D., Tietze C., Alpers D. H., Brasitus T. A. Effects of 1,25-dihydroxyvitamin D3 on proliferation and differentiation of Caco-2 cells. Endocrinology. 1994 Apr;134(4):1710–1717. doi: 10.1210/endo.134.4.8137734. [DOI] [PubMed] [Google Scholar]
- Hermiston M. L., Gordon J. I. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995 Nov 17;270(5239):1203–1207. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- Iiboshi Y., Nezu R., Kennedy M., Fujii M., Wasa M., Fukuzawa M., Kamata S., Takagi Y., Okada A. Total parenteral nutrition decreases luminal mucous gel and increases permeability of small intestine. JPEN J Parenter Enteral Nutr. 1994 Jul-Aug;18(4):346–350. doi: 10.1177/014860719401800412. [DOI] [PubMed] [Google Scholar]
- Jacobs L. R., Schneeman B. O. Effects of dietary wheat bran on rat colonic structure and mucosal cell growth. J Nutr. 1981 May;111(5):798–803. doi: 10.1093/jn/111.5.798. [DOI] [PubMed] [Google Scholar]
- Kripke S. A., Fox A. D., Berman J. M., Settle R. G., Rombeau J. L. Stimulation of intestinal mucosal growth with intracolonic infusion of short-chain fatty acids. JPEN J Parenter Enteral Nutr. 1989 Mar-Apr;13(2):109–116. doi: 10.1177/0148607189013002109. [DOI] [PubMed] [Google Scholar]
- Lev R., Griffiths W. C. Colonic and small intestinal alkaline phosphatase. A histochemical and biochemical study. Gastroenterology. 1982 Jun;82(6):1427–1435. [PubMed] [Google Scholar]
- Luciano L., Reale E., Rechkemmer G., von Engelhardt W. Structure of zonulae occludentes and the permeability of the epithelium to short-chain fatty acids in the proximal and the distal colon of guinea pig. J Membr Biol. 1984;82(2):145–156. doi: 10.1007/BF01868939. [DOI] [PubMed] [Google Scholar]
- Madara J. L., Trier J. S., Neutra M. R. Structural changes in the plasma membrane accompanying differentiation of epithelial cells in human and monkey small intestine. Gastroenterology. 1980 May;78(5 Pt 1):963–975. [PubMed] [Google Scholar]
- Marcial M. A., Carlson S. L., Madara J. L. Partitioning of paracellular conductance along the ileal crypt-villus axis: a hypothesis based on structural analysis with detailed consideration of tight junction structure-function relationships. J Membr Biol. 1984;80(1):59–70. doi: 10.1007/BF01868690. [DOI] [PubMed] [Google Scholar]
- Mariadason J. M., Barkla D. H., Gibson P. R. Effect of short-chain fatty acids on paracellular permeability in Caco-2 intestinal epithelium model. Am J Physiol. 1997 Apr;272(4 Pt 1):G705–G712. doi: 10.1152/ajpgi.1997.272.4.G705. [DOI] [PubMed] [Google Scholar]
- Maroux S., Louvard D., Baratti J. The aminopeptidase from hog intestinal brush border. Biochim Biophys Acta. 1973 Sep 15;321(1):282–295. doi: 10.1016/0005-2744(73)90083-1. [DOI] [PubMed] [Google Scholar]
- McIntyre A., Gibson P. R., Young G. P. Butyrate production from dietary fibre and protection against large bowel cancer in a rat model. Gut. 1993 Mar;34(3):386–391. doi: 10.1136/gut.34.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre A., Young G. P., Taranto T., Gibson P. R., Ward P. B. Different fibers have different regional effects on luminal contents of rat colon. Gastroenterology. 1991 Nov;101(5):1274–1281. doi: 10.1016/0016-5085(91)90077-x. [DOI] [PubMed] [Google Scholar]
- Medina V., Afonso J. J., Alvarez-Arguelles H., Hernández C., González F. Sodium butyrate inhibits carcinoma development in a 1,2-dimethylhydrazine-induced rat colon cancer. JPEN J Parenter Enteral Nutr. 1998 Jan-Feb;22(1):14–17. doi: 10.1177/014860719802200114. [DOI] [PubMed] [Google Scholar]
- Reeves P. G. AIN-76 diet: should we change the formulation? J Nutr. 1989 Aug;119(8):1081–1082. doi: 10.1093/jn/119.8.1081. [DOI] [PubMed] [Google Scholar]
- Sadowski D. C., Meddings J. B. Luminal nutrients alter tight-junction permeability in the rat jejunum: an in vivo perfusion model. Can J Physiol Pharmacol. 1993 Oct-Nov;71(10-11):835–839. doi: 10.1139/y93-125. [DOI] [PubMed] [Google Scholar]
- Saunders P. R., Kosecka U., McKay D. M., Perdue M. H. Acute stressors stimulate ion secretion and increase epithelial permeability in rat intestine. Am J Physiol. 1994 Nov;267(5 Pt 1):G794–G799. doi: 10.1152/ajpgi.1994.267.5.G794. [DOI] [PubMed] [Google Scholar]
- Stragand J. J., Hagemann R. F. Dietary influence on colonic cell renewal. Am J Clin Nutr. 1977 Jun;30(6):918–923. doi: 10.1093/ajcn/30.6.918. [DOI] [PubMed] [Google Scholar]
- Sundqvist T., Lindström F., Magnusson K. E., Sköldstam L., Stjernström I., Tagesson C. Influence of fasting on intestinal permeability and disease activity in patients with rheumatoid arthritis. Scand J Rheumatol. 1982;11(1):33–38. doi: 10.3109/03009748209098111. [DOI] [PubMed] [Google Scholar]
- Tasman-Jones C., Owen R. L., Jones A. L. Semipurified dietary fiber and small-bowel morphology in rats. Dig Dis Sci. 1982 Jun;27(6):519–524. doi: 10.1007/BF01296731. [DOI] [PubMed] [Google Scholar]
- Walker W. A., Sanderson I. R. Epithelial barrier function to antigens. An overview. Ann N Y Acad Sci. 1992;664:10–17. doi: 10.1111/j.1749-6632.1992.tb39744.x. [DOI] [PubMed] [Google Scholar]
- Wang Q., Wang X. D., Jeppsson B., Andersson R., Karlsson B., Weström B. Influence of colostomy on in vivo and in vitro permeability of the rat colon. Dis Colon Rectum. 1996 Jun;39(6):663–670. doi: 10.1007/BF02056947. [DOI] [PubMed] [Google Scholar]
- Young G. P., Macrae F. A., Gibson P. R., Alexeyeff M., Whitehead R. H. Brush border hydrolases in normal and neoplastic colonic epithelium. J Gastroenterol Hepatol. 1992 Jul-Aug;7(4):347–354. doi: 10.1111/j.1440-1746.1992.tb00995.x. [DOI] [PubMed] [Google Scholar]
- Young G. P., McIntyre A., Albert V., Folino M., Muir J. G., Gibson P. R. Wheat bran suppresses potato starch--potentiated colorectal tumorigenesis at the aberrant crypt stage in a rat model. Gastroenterology. 1996 Feb;110(2):508–514. doi: 10.1053/gast.1996.v110.pm8566598. [DOI] [PubMed] [Google Scholar]
- Young G. P., Rose I. S., Cropper S., Seetharam S., Alpers D. H. Hepatic clearance of rat plasma intestinal alkaline phosphatase. Am J Physiol. 1984 Oct;247(4 Pt 1):G419–G426. doi: 10.1152/ajpgi.1984.247.4.G419. [DOI] [PubMed] [Google Scholar]
- van der Hulst R. R., van Kreel B. K., von Meyenfeldt M. F., Brummer R. J., Arends J. W., Deutz N. E., Soeters P. B. Glutamine and the preservation of gut integrity. Lancet. 1993 May 29;341(8857):1363–1365. doi: 10.1016/0140-6736(93)90939-e. [DOI] [PubMed] [Google Scholar]