Abstract
BACKGROUND/AIMS—Most patients infected with hepatitis C virus (HCV) develop chronic infection and persistent viraemia. The immune mechanisms responsible for resolution of viraemia remain poorly understood. HCV specific humoral and cellular immune responses in patients with and without viraemia were investigated. METHODS—In vitro T helper (TH) lymphocyte responses to structural and non-structural HCV proteins were determined by means of proliferative response and cytokine production in 35 anti-HCV positive/HCV RNA negative patients and in 31 patients with chronic HCV infection and persistent viraemia. Humoral responses were determined by measuring HCV specific antibody quantity and specificity. RESULTS—A TH response to two or more HCV proteins was present in 18 of 35 patients with serological viral clearance compared with just one of 31 viraemic patients (p = 0.00001). HCV specific interferon-γ production was increased only in the former group. In contrast, the antibody levels were significantly lower and directed at fewer HCV antigens in patients with undetectable HCV RNA. CONCLUSIONS—Patients without viraemia after HCV infection frequently have strong TH lymphocyte responses of the TH1 type to multiple HCV antigens many years after the onset of infection, whereas antibody responses are less marked. These results suggest that control of HCV replication may depend on effective TH lymphocyte activation.
Keywords: hepatitis C virus; liver; Th1/Th2 cells; T helper cytokines; viral clearance
Full Text
The Full Text of this article is available as a PDF (155.6 KB).
Figure 1 .
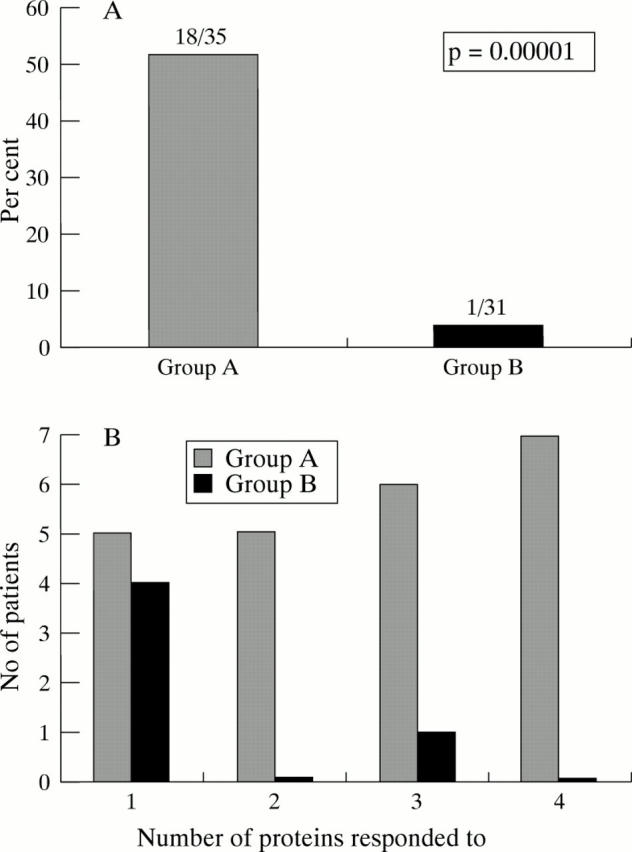
Presence of significant TH cell proliferative response to hepatitis C virus (HCV) antigens in patients with viral clearance (group A) compared with that in patients with viral persistence (group B). (A) Percentage of patients in groups A and B with a significant response to two or more HCV proteins. Numbers above bars are numbers responding/numbers tested. p value was calculated using the χ2 test. (B) Number of patients in each group with a significant response to various numbers of HCV proteins.
Figure 2 .
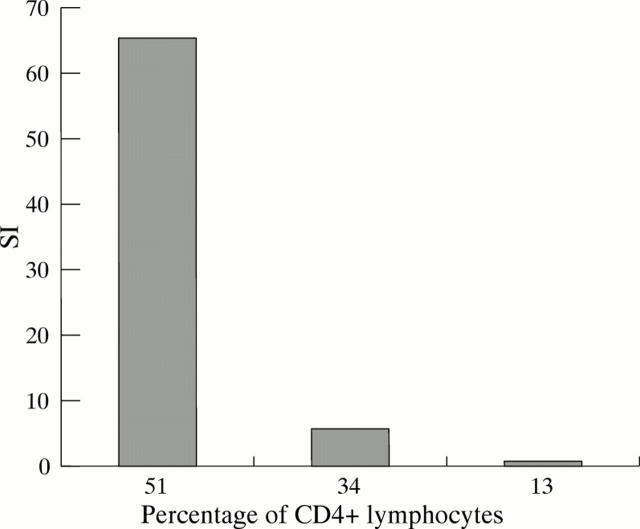
Effect of CD4 cell depletion on response to hepatitis C virus (HCV) non-structural protein 4 (NS4); confirmation that the proliferative response to HCV antigens is restricted to CD4 lymphocytes. Progressive depletion of CD4 lymphocytes from the pool of peripheral blood mononuclear cells progressively reduces the stimulation index (SI) to NS4 in a typical case.
Figure 3 .
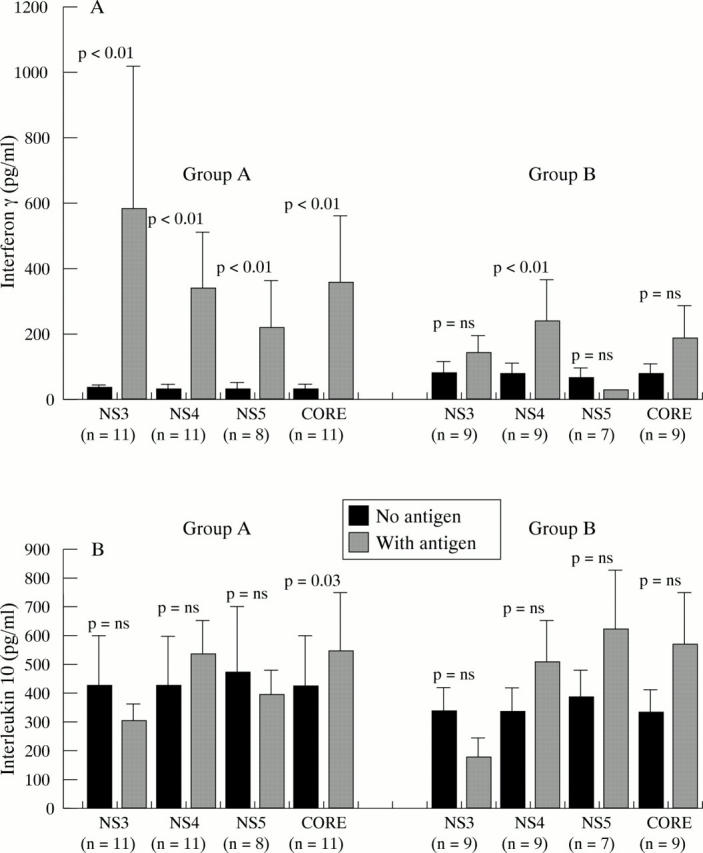
Comparison of in vitro production of interferon-γ (A) and interleukin-10 (B) in response to hepatitis C virus (HCV) proteins in patients with viral clearance (group A) and patients with viral persistence (group B). Bars indicate mean concentration of cytokine produced by lymphocytes cultured in the absence of HCV proteins (no anitgen) or in the presence of HCV proteins (with antigen). Vertical lines represent standard error. p values were calculated using the Wilcoxon test on all values.
Figure 4 .
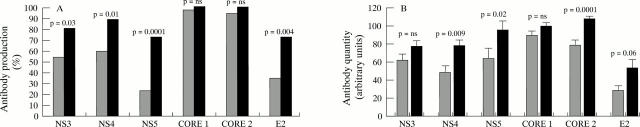
Comparison between groups A (shaded bars) and B (solid bars) of antibody responses to individual hepatitis C virus (HCV) antigens as measured by LIA-Scan. (A) The percentage of patients with detectable antibody to each HCV antigen. p values were calculated using the χ2 test. (B) Quantification of serum antibody levels in arbitrary units to HCV non-structural protein (NS)3, NS4, NS5, core 1, core 2, and E2. Bars represent mean values from all those with detectable antibody, excluding values of zero or below the cut off value. Vertical lines represent standard error. p values were calculated using the Mann-Whitney U test for unpaired samples. Core 1 and core 2 are non-overlapping epitope clusters from HCV core. E2 refers to envelope 2/hypervariable region 1.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter H. J., Purcell R. H., Shih J. W., Melpolder J. C., Houghton M., Choo Q. L., Kuo G. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989 Nov 30;321(22):1494–1500. doi: 10.1056/NEJM198911303212202. [DOI] [PubMed] [Google Scholar]
- Alter H. J. To C or not to C: these are the questions. Blood. 1995 Apr 1;85(7):1681–1695. [PubMed] [Google Scholar]
- Alter M. J., Margolis H. S., Krawczynski K., Judson F. N., Mares A., Alexander W. J., Hu P. Y., Miller J. K., Gerber M. A., Sampliner R. E. The natural history of community-acquired hepatitis C in the United States. The Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. N Engl J Med. 1992 Dec 31;327(27):1899–1905. doi: 10.1056/NEJM199212313272702. [DOI] [PubMed] [Google Scholar]
- Battegay M., Fikes J., Di Bisceglie A. M., Wentworth P. A., Sette A., Celis E., Ching W. M., Grakoui A., Rice C. M., Kurokohchi K. Patients with chronic hepatitis C have circulating cytotoxic T cells which recognize hepatitis C virus-encoded peptides binding to HLA-A2.1 molecules. J Virol. 1995 Apr;69(4):2462–2470. doi: 10.1128/jvi.69.4.2462-2470.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botarelli P., Brunetto M. R., Minutello M. A., Calvo P., Unutmaz D., Weiner A. J., Choo Q. L., Shuster J. R., Kuo G., Bonino F. T-lymphocyte response to hepatitis C virus in different clinical courses of infection. Gastroenterology. 1993 Feb;104(2):580–587. doi: 10.1016/0016-5085(93)90430-k. [DOI] [PubMed] [Google Scholar]
- Boyer J. D., Ugen K. E., Wang B., Agadjanyan M., Gilbert L., Bagarazzi M. L., Chattergoon M., Frost P., Javadian A., Williams W. V. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. Nat Med. 1997 May;3(5):526–532. doi: 10.1038/nm0597-526. [DOI] [PubMed] [Google Scholar]
- Clerici M., Shearer G. M. The Th1-Th2 hypothesis of HIV infection: new insights. Immunol Today. 1994 Dec;15(12):575–581. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Conry-Cantilena C., VanRaden M., Gibble J., Melpolder J., Shakil A. O., Viladomiu L., Cheung L., DiBisceglie A., Hoofnagle J., Shih J. W. Routes of infection, viremia, and liver disease in blood donors found to have hepatitis C virus infection. N Engl J Med. 1996 Jun 27;334(26):1691–1696. doi: 10.1056/NEJM199606273342602. [DOI] [PubMed] [Google Scholar]
- Cramp M. E., Carucci P., Underhill J., Naoumov N. V., Williams R., Donaldson P. T. Association between HLA class II genotype and spontaneous clearance of hepatitis C viraemia. J Hepatol. 1998 Aug;29(2):207–213. doi: 10.1016/s0168-8278(98)80005-6. [DOI] [PubMed] [Google Scholar]
- Del Prete G., Maggi E., Romagnani S. Human Th1 and Th2 cells: functional properties, mechanisms of regulation, and role in disease. Lab Invest. 1994 Mar;70(3):299–306. [PubMed] [Google Scholar]
- Diepolder H. M., Zachoval R., Hoffmann R. M., Wierenga E. A., Santantonio T., Jung M. C., Eichenlaub D., Pape G. R. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet. 1995 Oct 14;346(8981):1006–1007. doi: 10.1016/s0140-6736(95)91691-1. [DOI] [PubMed] [Google Scholar]
- Farci P., Alter H. J., Govindarajan S., Wong D. C., Engle R., Lesniewski R. R., Mushahwar I. K., Desai S. M., Miller R. H., Ogata N. Lack of protective immunity against reinfection with hepatitis C virus. Science. 1992 Oct 2;258(5079):135–140. doi: 10.1126/science.1279801. [DOI] [PubMed] [Google Scholar]
- Farci P., Alter H. J., Wong D. C., Miller R. H., Govindarajan S., Engle R., Shapiro M., Purcell R. H. Prevention of hepatitis C virus infection in chimpanzees after antibody-mediated in vitro neutralization. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7792–7796. doi: 10.1073/pnas.91.16.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerken G., Pontisso P., Roggendorf M., Grazia Rumi M., Simmonds P., Trepo C., Zeuzem S., Colucci G. Clinical evaluation of a single reaction, diagnostic polymerase chain reaction assay for the detection of hepatitis C virus RNA. J Hepatol. 1996 Jan;24(1):33–37. doi: 10.1016/s0168-8278(96)80183-8. [DOI] [PubMed] [Google Scholar]
- Haydon G. H., Jarvis L. M., Blair C. S., Simmonds P., Harrison D. J., Simpson K. J., Hayes P. C. Clinical significance of intrahepatic hepatitis C virus levels in patients with chronic HCV infection. Gut. 1998 Apr;42(4):570–575. doi: 10.1136/gut.42.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann R. M., Diepolder H. M., Zachoval R., Zwiebel F. M., Jung M. C., Scholz S., Nitschko H., Riethmüller G., Pape G. R. Mapping of immunodominant CD4+ T lymphocyte epitopes of hepatitis C virus antigens and their relevance during the course of chronic infection. Hepatology. 1995 Mar;21(3):632–638. [PubMed] [Google Scholar]
- Klassen L. W., Tuma D., Sorrell M. F. Immune mechanisms of alcohol-induced liver disease. Hepatology. 1995 Jul;22(1):355–357. [PubMed] [Google Scholar]
- Lai M. E., Mazzoleni A. P., Argiolu F., De Virgilis S., Balestrieri A., Purcell R. H., Cao A., Farci P. Hepatitis C virus in multiple episodes of acute hepatitis in polytransfused thalassaemic children. Lancet. 1994 Feb 12;343(8894):388–390. doi: 10.1016/s0140-6736(94)91224-6. [DOI] [PubMed] [Google Scholar]
- Lechmann M., Ihlenfeldt H. G., Braunschweiger I., Giers G., Jung G., Matz B., Kaiser R., Sauerbruch T., Spengler U. T- and B-cell responses to different hepatitis C virus antigens in patients with chronic hepatitis C infection and in healthy anti-hepatitis C virus--positive blood donors without viremia. Hepatology. 1996 Oct;24(4):790–795. doi: 10.1002/hep.510240406. [DOI] [PubMed] [Google Scholar]
- Marinos G., Torre F., Chokshi S., Hussain M., Clarke B. E., Rowlands D. J., Eddleston A. L., Naoumov N. V., Williams R. Induction of T-helper cell response to hepatitis B core antigen in chronic hepatitis B: a major factor in activation of the host immune response to the hepatitis B virus. Hepatology. 1995 Oct;22(4 Pt 1):1040–1049. doi: 10.1016/0270-9139(95)90607-x. [DOI] [PubMed] [Google Scholar]
- McDonnell W. M., Askari F. K. DNA vaccines. N Engl J Med. 1996 Jan 4;334(1):42–45. doi: 10.1056/NEJM199601043340110. [DOI] [PubMed] [Google Scholar]
- Michalak T. I., Pasquinelli C., Guilhot S., Chisari F. V. Hepatitis B virus persistence after recovery from acute viral hepatitis. J Clin Invest. 1994 Jan;93(1):230–239. doi: 10.1172/JCI116950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minutello M. A., Pileri P., Unutmaz D., Censini S., Kuo G., Houghton M., Brunetto M. R., Bonino F., Abrignani S. Compartmentalization of T lymphocytes to the site of disease: intrahepatic CD4+ T cells specific for the protein NS4 of hepatitis C virus in patients with chronic hepatitis C. J Exp Med. 1993 Jul 1;178(1):17–25. doi: 10.1084/jem.178.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale G., Bertoni R., Lamonaca V., Valli A., Massari M., Mori C., Rumi M. G., Houghton M., Fiaccadori F., Ferrari C. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J Clin Invest. 1996 Aug 1;98(3):706–714. doi: 10.1172/JCI118842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Penna A., Artini M., Cavalli A., Levrero M., Bertoletti A., Pilli M., Chisari F. V., Rehermann B., Del Prete G., Fiaccadori F. Long-lasting memory T cell responses following self-limited acute hepatitis B. J Clin Invest. 1996 Sep 1;98(5):1185–1194. doi: 10.1172/JCI118902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehermann B., Chang K. M., McHutchison J. G., Kokka R., Houghton M., Chisari F. V. Quantitative analysis of the peripheral blood cytotoxic T lymphocyte response in patients with chronic hepatitis C virus infection. J Clin Invest. 1996 Sep 15;98(6):1432–1440. doi: 10.1172/JCI118931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehermann B., Ferrari C., Pasquinelli C., Chisari F. V. The hepatitis B virus persists for decades after patients' recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996 Oct;2(10):1104–1108. doi: 10.1038/nm1096-1104. [DOI] [PubMed] [Google Scholar]
- Rossol S., Voth R., Laubenstein H. P., Müller W. E., Schröder H. C., Meyer zum Büschenfelde K. H., Hess G. Interferon production in patients infected with HIV-1. J Infect Dis. 1989 May;159(5):815–821. doi: 10.1093/infdis/159.5.815. [DOI] [PubMed] [Google Scholar]
- Rümenapf T., Stark R., Meyers G., Thiel H. J. Structural proteins of hog cholera virus expressed by vaccinia virus: further characterization and induction of protective immunity. J Virol. 1991 Feb;65(2):589–597. doi: 10.1128/jvi.65.2.589-597.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger J. J., Brandriss M. W., Cropp C. B., Monath T. P. Protection against yellow fever in monkeys by immunization with yellow fever virus nonstructural protein NS1. J Virol. 1986 Dec;60(3):1153–1155. doi: 10.1128/jvi.60.3.1153-1155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A., Gazzinelli R. T., Oswald I. P., Clerici M., Kullberg M., Pearce E. J., Berzofsky J. A., Mosmann T. R., James S. L., Morse H. C., 3rd Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol Rev. 1992 Jun;127:183–204. doi: 10.1111/j.1600-065x.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- Thursz M. R., Kwiatkowski D., Allsopp C. E., Greenwood B. M., Thomas H. C., Hill A. V. Association between an MHC class II allele and clearance of hepatitis B virus in the Gambia. N Engl J Med. 1995 Apr 20;332(16):1065–1069. doi: 10.1056/NEJM199504203321604. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- Yuki N., Hayashi N., Kasahara A., Hagiwara H., Mita E., Ohkawa K., Katayama K., Fusamoto H., Kamada T. Quantitative analysis of antibody to hepatitis C virus envelope 2 glycoprotein in patients with chronic hepatitis C virus infection. Hepatology. 1996 May;23(5):947–952. doi: 10.1053/jhep.1996.v23.pm0008621173. [DOI] [PubMed] [Google Scholar]
- van der Poel C. L., Cuypers H. T., Reesink H. W. Hepatitis C virus six years on. Lancet. 1994 Nov 26;344(8935):1475–1479. doi: 10.1016/s0140-6736(94)90293-3. [DOI] [PubMed] [Google Scholar]