Abstract
BACKGROUND—The response of the oesophagus to refluxed gastric contents is likely to depend on intact neural mechanisms in the oesophageal mucosa. The epithelial innervation has not been systematically evaluated in health or reflux disease. AIMS—To study oesophageal epithelial innervation in controls, and also inflamed and non-inflamed mucosa in patients with reflux oesophagitis and healed oesophagitis. PATIENTS—Ten controls, nine patients with reflux oesophagitis, and five patients with healed oesophagitis. METHODS—Oesophageal epithelial biopsy specimens were obtained at endoscopy. The distribution of the neuronal marker protein gene product 9.5 (PGP), and the neuropeptides calcitonin gene related peptide (CGRP), neuropeptide Y (NPY), substance P (SP), and vasoactive intestinal peptide (VIP) were investigated by immunohistochemistry. Density of innervation was assessed by the proportion of papillae in each oesophageal epithelial biopsy specimen containing immunoreactive fibres (found in the subepithelium and epithelial papillae, but not penetrating the epithelium). RESULTS—The proportion of papillae positive for PGP immunoreactive nerve fibres was significantly increased in inflamed tissue when compared with controls, and non-inflamed and healed tissue. There was also a significant increase in VIP immunoreactive fibres within epithelial papillae. Other neuropeptides showed no proportional changes in inflammation. CONCLUSIONS—Epithelial biopsy specimens can be used to assess innervation in the oesophagus. The innervation of the oesophageal mucosa is not altered in non-inflamed tissue of patients with oesophagitis but alters in response to inflammation, where there is a selective increase (about three- to fourfold) in VIP containing nerves.
Keywords: gastro-oesophageal reflux; reflux oesophagitis; innervation; neuropeptides; inflammation
Full Text
The Full Text of this article is available as a PDF (140.5 KB).
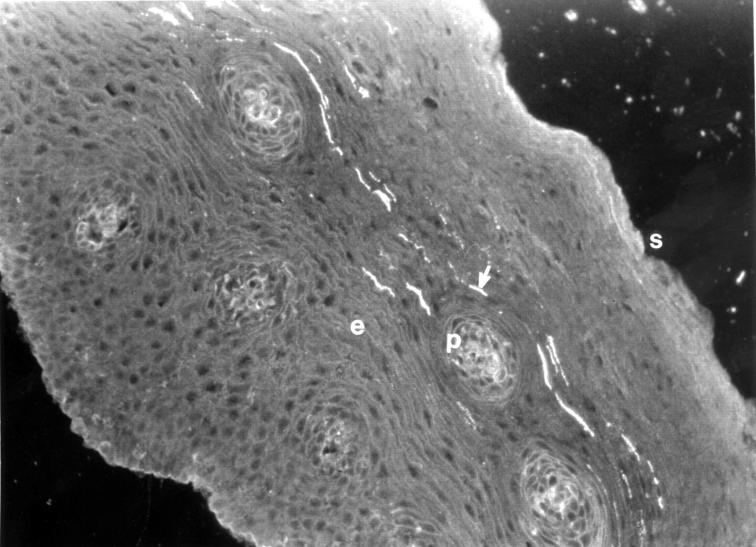
Figure 1 .
A photomicrograph of an oesophageal biopsy section from the inflamed oesophageal epithelium from a subject with reflux oesophagitis stained for PGP. The luminal surface is indicated (s). The papillae (p) extend into the epithelium (e). Immunofluorescent staining is seen in the subepithelial plexus extending across the upper poles of most of the papillae (arrow). Original magnification ×200.
Figure 2 .
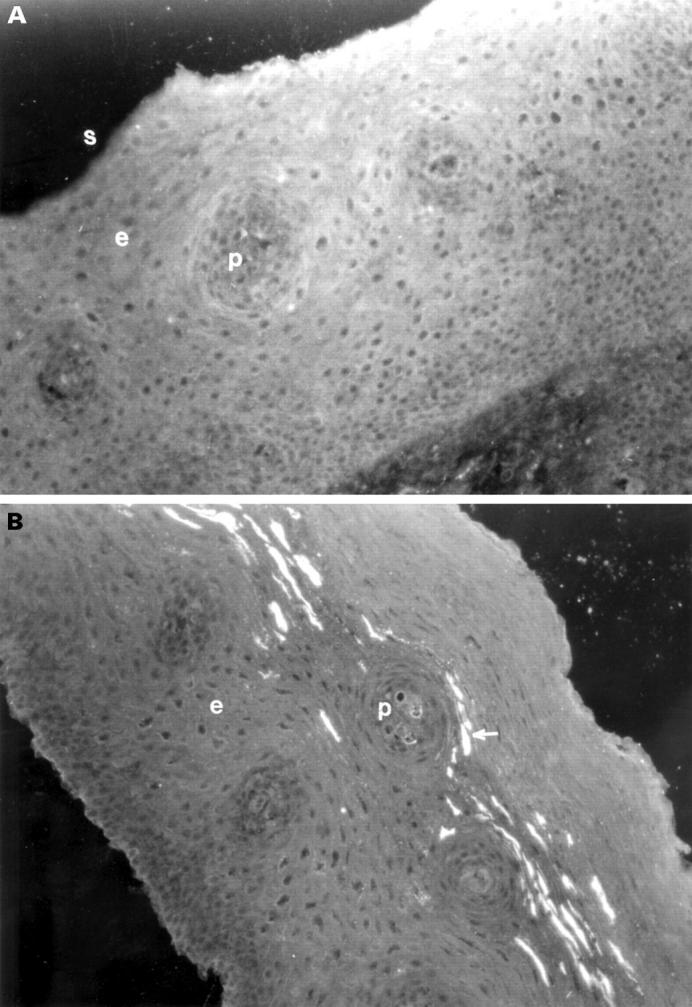
(A) Photomicrograph of an oesophageal biopsy section from control tissue stained for VIP. The luminal surface is indicated (s). The papillae (p) extend into the epithelium (e). There are no nerves staining positive for VIP in this biopsy specimen. (B) Photomicrograph of an oesophageal biopsy section from the inflamed oesophageal epithelium of a subject with reflux oesophagitis stained for VIP. The papillae (p) extend into the epithelium (e). Immunofluorescent staining is seen in the subepithelial plexus extending across the upper poles of most of the papillae (arrow). Original magnification ×200.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahtaridis G., Snape W. J., Jr, Cohen S. Lower esophageal sphincter pressure as an index of gastroesophageal acid reflux. Dig Dis Sci. 1981 Nov;26(11):993–998. doi: 10.1007/BF01314761. [DOI] [PubMed] [Google Scholar]
- Baldi F., Ferrarini F., Balestra R., Borioni D., Longanesi A., Miglioli M., Barbara L. Oesophageal motor events at the occurrence of acid reflux and during endogenous acid exposure in healthy subjects and in patients with oesophagitis. Gut. 1985 Apr;26(4):336–341. doi: 10.1136/gut.26.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass B. L., Schweitzer E. J., Harmon J. W., Kraimer J. H+ back diffusion interferes with intrinsic reactive regulation of esophageal mucosal blood flow. Surgery. 1984 Aug;96(2):404–413. [PubMed] [Google Scholar]
- Belai A., Boulos P. B., Robson T., Burnstock G. Neurochemical coding in the small intestine of patients with Crohn's disease. Gut. 1997 Jun;40(6):767–774. doi: 10.1136/gut.40.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biancani P., Billett G., Hillemeier C., Nissensohn M., Rhim B. Y., Szewczak S., Behar J. Acute experimental esophagitis impairs signal transduction in cat lower esophageal sphincter circular muscle. Gastroenterology. 1992 Oct;103(4):1199–1206. doi: 10.1016/0016-5085(92)91504-w. [DOI] [PubMed] [Google Scholar]
- Binder H. J., Lemp G. F., Gardner J. D. Receptors for vasoactive intestinal peptide and secretin on small intestinal epithelial cells. Am J Physiol. 1980 Mar;238(3):G190–G196. doi: 10.1152/ajpgi.1980.238.3.G190. [DOI] [PubMed] [Google Scholar]
- Bishop A. E., Polak J. M., Bryant M. G., Bloom S. R., Hamilton S. Abnormalities of vasoactive intestinal polypeptide-containing nerves in Crohn's disease. Gastroenterology. 1980 Nov;79(5 Pt 1):853–860. [PubMed] [Google Scholar]
- Bontempo I., Piretta L., Corazziari E., Michetti F., Anzini F., Torsoli A. Effects of intraluminal acidification on oesophageal motor activity. Gut. 1994 Jul;35(7):884–890. doi: 10.1136/gut.35.7.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P., Silman A. Statistical methods for assessing observer variability in clinical measures. BMJ. 1992 Jun 6;304(6840):1491–1494. doi: 10.1136/bmj.304.6840.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. M., Snowdon C. F., Slee B., Sandle L. N., Rees W. D. Measurement of bicarbonate output from the intact human oesophagus. Gut. 1993 Jul;34(7):872–880. doi: 10.1136/gut.34.7.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corazziari E., Bontempo I., Anzini F., Torsoli A. Motor activity of the distal oesophagus and gastrooesophageal reflux. Gut. 1984 Jan;25(1):7–13. doi: 10.1136/gut.25.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmsathaphorn K., Harms V., Yamashiro D. J., Hughes R. J., Binder H. J., Wright E. M. Preferential binding of vasoactive intestinal polypeptide to basolateral membrane of rat and rabbit enterocytes. J Clin Invest. 1983 Jan;71(1):27–35. doi: 10.1172/JCI110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S. K., Matossian H. B., Meirowitz R. F., Vaeth J. Modulation of salivary secretion by acid infusion in the distal esophagus in humans. Gastroenterology. 1992 Dec;103(6):1833–1841. doi: 10.1016/0016-5085(92)91442-7. [DOI] [PubMed] [Google Scholar]
- Eastwood G. L., Castell D. O., Higgs R. H. Experimental esophagitis in cats impairs lower esophageal sphincter pressure. Gastroenterology. 1975 Jul;69(1):146–153. [PubMed] [Google Scholar]
- Eysselein V. E., Reinshagen M., Cominelli F., Sternini C., Davis W., Patel A., Nast C. C., Bernstein D., Anderson K., Khan H. Calcitonin gene-related peptide and substance P decrease in the rabbit colon during colitis. A time study. Gastroenterology. 1991 Nov;101(5):1211–1219. doi: 10.1016/0016-5085(91)90069-w. [DOI] [PubMed] [Google Scholar]
- Ferri G. L., Adrian T. E., Soimero L., Blank M., Cavalli D., Biliotti G., Polak J. M., Bloom S. R. Intramural distribution of immunoreactive vasoactive intestinal polypeptide (VIP), substance P, somatostatin and mammalian bombesin in the oesophago-gastro-pyloric region of the human gut. Cell Tissue Res. 1989 Apr;256(1):191–197. doi: 10.1007/BF00224734. [DOI] [PubMed] [Google Scholar]
- Ferri G. L., Botti P., Biliotti G., Rebecchi L., Bloom S. R., Tonelli L., Labò G., Polak J. M. VIP-, substance P- and met-enkephalin-immunoreactive innervation of the human gastroduodenal mucosa and Brunner's glands. Gut. 1984 Sep;25(9):948–952. doi: 10.1136/gut.25.9.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin E., Karmeli F., Selinger Z., Rachmilewitz D. Colonic substance P levels are increased in ulcerative colitis and decreased in chronic severe constipation. Dig Dis Sci. 1989 May;34(5):754–757. doi: 10.1007/BF01540348. [DOI] [PubMed] [Google Scholar]
- Greenwood B., Mantle M. Mucin and protein release in the rabbit jejunum: effects of bethanechol and vagal nerve stimulation. Gastroenterology. 1992 Aug;103(2):496–505. doi: 10.1016/0016-5085(92)90839-q. [DOI] [PubMed] [Google Scholar]
- Hamilton B. H., Orlando R. C. In vivo alkaline secretion by mammalian esophagus. Gastroenterology. 1989 Sep;97(3):640–648. doi: 10.1016/0016-5085(89)90635-5. [DOI] [PubMed] [Google Scholar]
- Higgs R. H., Castell D. O., Eastwood G. L. Studies on the mechanism of esophagitis-induced lower esophageal sphincter hypotension in cats. Gastroenterology. 1976 Jul;71(1):51–56. [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988 Mar;24(3):739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Holzer P., Pabst M. A., Lippe I. T. Intragastric capsaicin protects against aspirin-induced lesion formation and bleeding in the rat gastric mucosa. Gastroenterology. 1989 Jun;96(6):1425–1433. doi: 10.1016/0016-5085(89)90508-8. [DOI] [PubMed] [Google Scholar]
- Hopwood D., Logan K. R., Coghill G., Bouchier I. A. Histochemical studies of mucosubstances and lipids in normal human oesophageal epithelium. Histochem J. 1977 Mar;9(2):153–161. doi: 10.1007/BF01003627. [DOI] [PubMed] [Google Scholar]
- Ismail-Beigi F., Horton P. F., Pope C. E., 2nd Histological consequences of gastroesophageal reflux in man. Gastroenterology. 1970 Feb;58(2):163–174. [PubMed] [Google Scholar]
- Ismail-Beigi F., Pope C. E., 2nd Distribution of the histological changes of gastroesophageal reflux in the distal esophagus of man. Gastroenterology. 1974 Jun;66(6):1109–1113. [PubMed] [Google Scholar]
- Johnson L. F., Demeester T. R., Haggitt R. C. Esophageal epithelial response to gastroesophageal reflux. A quantitative study. Am J Dig Dis. 1978 Jun;23(6):498–509. doi: 10.1007/BF01072693. [DOI] [PubMed] [Google Scholar]
- Keast J. R., Furness J. B., Costa M. Distribution of peptide-containing neurons and endocrine cells in the rabbit gastrointestinal tract, with particular reference to the mucosa. Cell Tissue Res. 1987 Jun;248(3):565–577. doi: 10.1007/BF00216485. [DOI] [PubMed] [Google Scholar]
- Keast J. R., Furness J. B., Costa M. Origins of peptide and norepinephrine nerves in the mucosa of the guinea pig small intestine. Gastroenterology. 1984 Apr;86(4):637–644. [PubMed] [Google Scholar]
- Koch T. R., Carney J. A., Go V. L. Distribution and quantitation of gut neuropeptides in normal intestine and inflammatory bowel diseases. Dig Dis Sci. 1987 Apr;32(4):369–376. doi: 10.1007/BF01296290. [DOI] [PubMed] [Google Scholar]
- Livstone E. M., Sheahan D. G., Behar J. Studies of esophageal epithelial cell proliferation in patients with reflux esophagitis. Gastroenterology. 1977 Dec;73(6):1315–1319. [PubMed] [Google Scholar]
- Mazumdar S., Das K. M. Immunocytochemical localization of vasoactive intestinal peptide and substance P in the colon from normal subjects and patients with inflammatory bowel disease. Am J Gastroenterol. 1992 Feb;87(2):176–181. [PubMed] [Google Scholar]
- McGregor G. P., Conlon J. M. Regulatory peptide and serotonin content and brush-border enzyme activity in the rat gastrointestinal tract following neonatal treatment with capsaicin; lack of effect on epithelial markers. Regul Pept. 1991 Feb 1;32(2):109–119. doi: 10.1016/0167-0115(91)90039-j. [DOI] [PubMed] [Google Scholar]
- Meleagros L., Ghatei M. A., Bloom S. R. Release of vasodilator, but not vasoconstrictor, neuropeptides and of enteroglucagon by intestinal ischaemia/reperfusion in the rat. Gut. 1994 Dec;35(12):1701–1706. doi: 10.1136/gut.35.12.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers R. L., Orlando R. C. In vivo bicarbonate secretion by human esophagus. Gastroenterology. 1992 Oct;103(4):1174–1178. doi: 10.1016/0016-5085(92)91501-t. [DOI] [PubMed] [Google Scholar]
- Milner P., Crowe R., Kamm M. A., Lennard-Jones J. E., Burnstock G. Vasoactive intestinal polypeptide levels in sigmoid colon in idiopathic constipation and diverticular disease. Gastroenterology. 1990 Sep;99(3):666–675. doi: 10.1016/0016-5085(90)90953-x. [DOI] [PubMed] [Google Scholar]
- Namiot Z., Rourk R. M., Piascik R., Hetzel D. P., Sarosiek J., McCallum R. W. Interrelationship between esophageal challenge with mechanical and chemical stimuli and salivary protective mechanisms. Am J Gastroenterol. 1994 Apr;89(4):581–587. [PubMed] [Google Scholar]
- Namiot Z., Sarosiek J., Marcinkiewicz M., Edmunds M. C., McCallum R. W. Declined human esophageal mucin secretion in patients with severe reflux esophagitis. Dig Dis Sci. 1994 Dec;39(12):2523–2529. doi: 10.1007/BF02087685. [DOI] [PubMed] [Google Scholar]
- Namiot Z., Sarosiek J., Rourk R. M., Hetzel D. P., McCallum R. W. Human esophageal secretion: mucosal response to luminal acid and pepsin. Gastroenterology. 1994 Apr;106(4):973–981. doi: 10.1016/0016-5085(94)90756-0. [DOI] [PubMed] [Google Scholar]
- Ny L., Alm P., Ekström P., Hannibal J., Larsson B., Andersson K. E. Nitric oxide synthase-containing, peptide-containing, and acetylcholinesterase-positive nerves in the cat lower oesophagus. Histochem J. 1994 Sep;26(9):721–733. doi: 10.1007/BF00158204. [DOI] [PubMed] [Google Scholar]
- O'Morain C., Bishop A. E., McGregor G. P., Levi A. J., Bloom S. R., Polak J. M., Peters T. J. Vasoactive intestinal peptide concentrations and immunocytochemical studies in rectal biopsies from patients with inflammatory bowel disease. Gut. 1984 Jan;25(1):57–61. doi: 10.1136/gut.25.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkman H. P., Reynolds J. C., Elfman K. S., Ogorek C. P. Calcitonin gene-related peptide: a sensory and motor neurotransmitter in the feline lower esophageal sphincter. Regul Pept. 1989 Apr;25(1):131–146. doi: 10.1016/0167-0115(89)90255-3. [DOI] [PubMed] [Google Scholar]
- Raybould H. E., Sternini C., Eysselein V. E., Yoneda M., Holzer P. Selective ablation of spinal afferent neurons containing CGRP attenuates gastric hyperemic response to acid. Peptides. 1992 Mar-Apr;13(2):249–254. doi: 10.1016/0196-9781(92)90104-b. [DOI] [PubMed] [Google Scholar]
- Reinshagen M., Patel A., Sottili M., Nast C., Davis W., Mueller K., Eysselein V. Protective function of extrinsic sensory neurons in acute rabbit experimental colitis. Gastroenterology. 1994 May;106(5):1208–1214. doi: 10.1016/0016-5085(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Reynolds J. C., Ouyang A., Cohen S. A lower esophageal sphincter reflex involving substance P. Am J Physiol. 1984 Apr;246(4 Pt 1):G346–G354. doi: 10.1152/ajpgi.1984.246.4.G346. [DOI] [PubMed] [Google Scholar]
- Robles-Chillida E. M., Rodrigo J., Mayo I., Arnedo A., Gómez A. Ultrastructure of free-ending nerve fibres in oesophageal epithelium. J Anat. 1981 Sep;133(Pt 2):227–233. [PMC free article] [PubMed] [Google Scholar]
- Rodrigo J., Hernández C. J., Vidal M. A., Pedrosa J. A. Vegetative innervation of the esophagus. III. Intraepithelial endings. Acta Anat (Basel) 1975;92(2):242–258. doi: 10.1159/000144444. [DOI] [PubMed] [Google Scholar]
- Salapatek A. M., Diamant N. E. Assessment of neural inhibition of the lower esophageal sphincter in cats with esophagitis. Gastroenterology. 1993 Mar;104(3):810–818. doi: 10.1016/0016-5085(93)91017-c. [DOI] [PubMed] [Google Scholar]
- Sarosiek J., Rourk R. M., Piascik R., Namiot Z., Hetzel D. P., McCallum R. W. The effect of esophageal mechanical and chemical stimuli on salivary mucin secretion in healthy individuals. Am J Med Sci. 1994 Jul;308(1):23–31. doi: 10.1097/00000441-199407000-00006. [DOI] [PubMed] [Google Scholar]
- Schwartz C. J., Kimberg D. V., Sheerin H. E., Field M., Said S. I. Vasoactive intestinal peptide stimulation of adenylate cyclase and active electrolyte secretion in intestinal mucosa. J Clin Invest. 1974 Sep;54(3):536–544. doi: 10.1172/JCI107790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scicchitano R., Biennenstock J., Stanisz A. M. In vivo immunomodulation by the neuropeptide substance P. Immunology. 1988 Apr;63(4):733–735. [PMC free article] [PubMed] [Google Scholar]
- Singaram C., Sengupta A., Sweet M. A., Sugarbaker D. J., Goyal R. K. Nitrinergic and peptidergic innervation of the human oesophagus. Gut. 1994 Dec;35(12):1690–1696. doi: 10.1136/gut.35.12.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjölund K., Schaffalitzky O. B., Muckadell D. E., Fahrenkrug J., Håkanson R., Peterson B. G., Sundler F. Peptide-containing nerve fibres in the gut wall in Crohn's disease. Gut. 1983 Aug;24(8):724–733. doi: 10.1136/gut.24.8.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternini C., Reeve J. R., Jr, Brecha N. Distribution and characterization of calcitonin gene-related peptide immunoreactivity in the digestive system of normal and capsaicin-treated rats. Gastroenterology. 1987 Oct;93(4):852–862. doi: 10.1016/0016-5085(87)90450-1. [DOI] [PubMed] [Google Scholar]
- Uddman R., Alumets J., Edvinsson L., Håkanson R., Sundler F. Peptidergic (VIP) innervation of the esophagus. Gastroenterology. 1978 Jul;75(1):5–8. [PubMed] [Google Scholar]
- Wattchow D. A., Furness J. B., Costa M. Distribution and coexistence of peptides in nerve fibers of the external muscle of the human gastrointestinal tract. Gastroenterology. 1988 Jul;95(1):32–41. doi: 10.1016/0016-5085(88)90287-9. [DOI] [PubMed] [Google Scholar]
- Wattchow D. A., Furness J. B., Costa M., O'Brien P. E., Peacock M. Distributions of neuropeptides in the human esophagus. Gastroenterology. 1987 Dec;93(6):1363–1371. doi: 10.1016/0016-5085(87)90267-8. [DOI] [PubMed] [Google Scholar]