Abstract
BACKGROUND—Intestinal morphology and function vary geographically. AIMS—These functions were assessed in asymptomatic volunteers in European, North American, Middle Eastern, Asian, African, and Caribbean countries. METHODS—Five hour urine collections were obtained from each subject following ingestion of a 100 ml iso-osmolar test solution containing 3-0-methyl-D-glucose, D-xylose, L-rhamnose, and lactulose after an overnight fast, to assess active (3-0-methyl-D-glucose) and passive (D-xylose) carrier mediated, and non-mediated (L-rhamnose) absorption capacity, as well as intestinal permeability (lactulose:rhamnose ratio). RESULTS—A comparison of results for subjects from tropical countries (n=218) with those resident in the combined temperate and subtropical region (Europe, United States, Qatar) (n=224) showed significant differences. Residents in tropical areas had a higher mean lactulose:rhamnose ratio and lower mean five hour recoveries of 3-0-methyl-D-glucose, D-xylose, and L-rhamnose, indicating higher intestinal permeability and lower absorptive capacity. Investigation of visiting residents suggested that differences in intestinal permeability and absorptive capacity were related to the area of residence. Subjects from Texas and Qatar, although comprised of several ethnic groups and resident in a subtropical area, showed no significant difference from European subjects. CONCLUSIONS—There are clearly demarcated variations in intestinal permeability and absorptive capacity affecting asymptomatic residents of different geographical areas which correspond with the condition described as tropical enteropathy. Results suggest the importance of environmental factors. The parameters investigated may be relevant to the predisposition of the indigenous population and travellers to diarrhoeal illness and malnutrition. Intestinal function in patients from the tropics may be difficult to interpret, but should take into account the range of values found in the asymptomatic normal population.
Keywords: intestinal permeability; absorption; tropical enteropathy; non-invasive sugar absorption/permeability test
Full Text
The Full Text of this article is available as a PDF (168.2 KB).
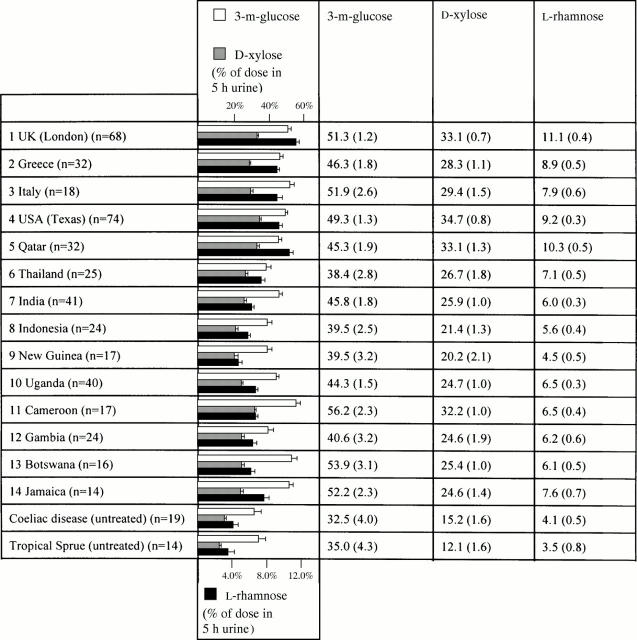
Figure 1 .
Monosaccharide absorption in "healthy" residents.
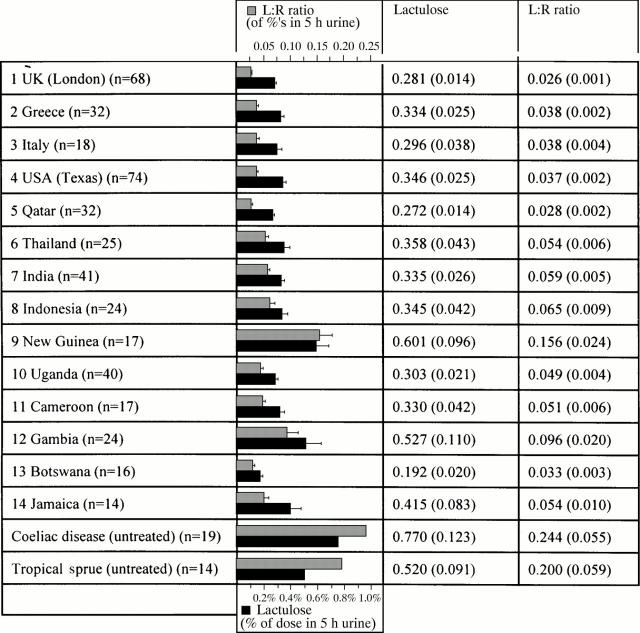
Figure 2 .
Intestinal permeability in "healthy" residents.
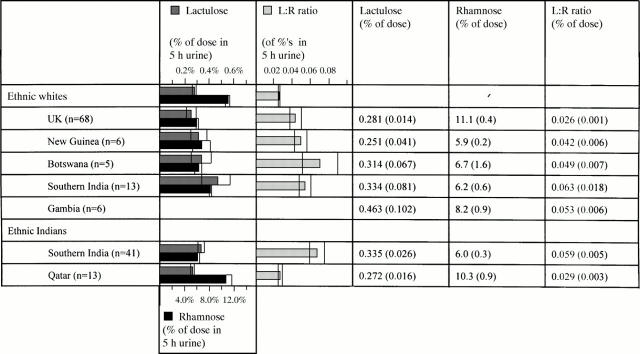
Figure 3 .
Intestinal absorption and permeability of specific ethnic groups resident in different geographical regions.
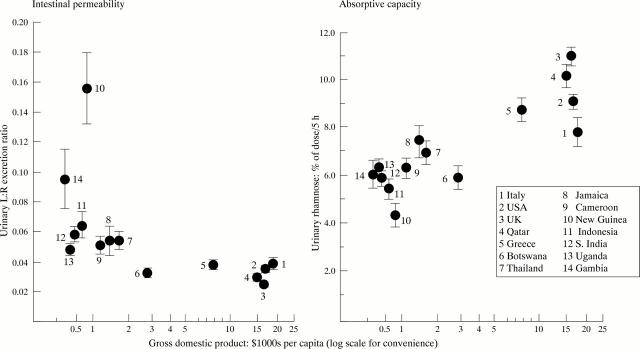
Figure 4 .
Correlation of intestinal permeability and absorptive capacity with gross domestic product per capita for country of residency plotted on a log scale.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behrens R. H., Taylor R. B., Low A. S., Warburton B., Pryce D. Traveller's diarrhoea; a controlled study of its effect on chloroquine and proguanil absorption. Trans R Soc Trop Med Hyg. 1994 Jan-Feb;88(1):86–88. doi: 10.1016/0035-9203(94)90513-4. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Levi S., Smethurst P., Menzies I. S., Levi A. J. Vindaloo and you. BMJ. 1988 Dec 24;297(6664):1629–1631. doi: 10.1136/bmj.297.6664.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I., MacPherson A., Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995 May;108(5):1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- Brunser O., Eidelman S., Klipstein F. A. Intestinal morphology of rural Haitians. A comparison between overt tropical sprue and asymptomatic subjects. Gastroenterology. 1970 May;58(5):655–668. [PubMed] [Google Scholar]
- Cook G. C., Menzies I. S. Intestinal absorption and unmediated permeation of sugars in post-infective tropical malabsorption (tropical sprue). Digestion. 1986;33(2):109–116. doi: 10.1159/000199282. [DOI] [PubMed] [Google Scholar]
- DuPont H. L., Capsuto E. G. Persistent diarrhea in travelers. Clin Infect Dis. 1996 Jan;22(1):124–128. doi: 10.1093/clinids/22.1.124. [DOI] [PubMed] [Google Scholar]
- DuPont H. L., Ericsson C. D. Prevention and treatment of traveler's diarrhea. N Engl J Med. 1993 Jun 24;328(25):1821–1827. doi: 10.1056/NEJM199306243282507. [DOI] [PubMed] [Google Scholar]
- FORDTRAN J. S., CLODI P. H., SOERGEL K. H., INGELFINGER F. J. Sugar absorption tests, with special reference to 3-0-methyl-d-glucose and d-xylose. Ann Intern Med. 1962 Dec;57:883–891. doi: 10.7326/0003-4819-57-6-883. [DOI] [PubMed] [Google Scholar]
- Falaiye J. M. Present status of subclinical intestinal malabsorption in the tropics. Br Med J. 1971 Nov 20;4(5785):454–458. doi: 10.1136/bmj.4.5785.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford R. P., Menzies I. S., Phillips A. D., Walker-Smith J. A., Turner M. W. Intestinal sugar permeability: relationship to diarrhoeal disease and small bowel morphology. J Pediatr Gastroenterol Nutr. 1985 Aug;4(4):568–574. [PubMed] [Google Scholar]
- Forget P., Sodoyez-Goffaux F., Zappitelli A. Permeability of the small intestine to [51Cr]EDTA in children with acute gastroenteritis or eczema. J Pediatr Gastroenterol Nutr. 1985 Jun;4(3):393–396. doi: 10.1097/00005176-198506000-00012. [DOI] [PubMed] [Google Scholar]
- Gerson C. D., Kent T. H., Saha J. R., Siddiqi N., Lindenbaum J. Recovery of small-intestinal structure and function after residence in the tropics. II. Studies in Indians and Pakistanis living in New York City. Ann Intern Med. 1971 Jul;75(1):41–48. doi: 10.7326/0003-4819-75-1-41. [DOI] [PubMed] [Google Scholar]
- Griffiths C. E., Menzies I. S., Barrison I. G., Leonard J. N., Fry L. Intestinal permeability in dermatitis herpetiformis. J Invest Dermatol. 1988 Aug;91(2):147–149. doi: 10.1111/1523-1747.ep12464390. [DOI] [PubMed] [Google Scholar]
- Iqbal T. H., Lewis K. O., Gearty J. C., Cooper B. T. Small intestinal permeability to mannitol and lactulose in the three ethnic groups resident in west Birmingham. Gut. 1996 Aug;39(2):199–203. doi: 10.1136/gut.39.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isolauri E., Juntunen M., Wiren S., Vuorinen P., Koivula T. Intestinal permeability changes in acute gastroenteritis: effects of clinical factors and nutritional management. J Pediatr Gastroenterol Nutr. 1989 May;8(4):466–473. doi: 10.1097/00005176-198905000-00008. [DOI] [PubMed] [Google Scholar]
- Keating J., Bjarnason I., Somasundaram S., Macpherson A., Francis N., Price A. B., Sharpstone D., Smithson J., Menzies I. S., Gazzard B. G. Intestinal absorptive capacity, intestinal permeability and jejunal histology in HIV and their relation to diarrhoea. Gut. 1995 Nov;37(5):623–629. doi: 10.1136/gut.37.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Falaiye J. M. Tropical sprue in expatriates from the tropics living in the continental United States. Medicine (Baltimore) 1969 Nov;48(6):475–491. doi: 10.1097/00005792-196948060-00003. [DOI] [PubMed] [Google Scholar]
- Lim S. G., Menzies I. S., Lee C. A., Johnson M. A., Pounder R. E. Intestinal permeability and function in patients infected with human immunodeficiency virus. A comparison with coeliac disease. Scand J Gastroenterol. 1993 Jul;28(7):573–580. doi: 10.3109/00365529309096090. [DOI] [PubMed] [Google Scholar]
- Lindenbaum J., Harmon J. W., Gerson C. D. Subclinical malabsorption in developing countries. Am J Clin Nutr. 1972 Oct;25(10):1056–1061. doi: 10.1093/ajcn/25.10.1056. [DOI] [PubMed] [Google Scholar]
- Lindenbaum J., Kent T. H., Sprinz H. Malabsorption and jejunitis in American Peace Corps volunteers in Pakistan. Ann Intern Med. 1966 Dec;65(6):1201–1209. doi: 10.7326/0003-4819-65-6-1201. [DOI] [PubMed] [Google Scholar]
- Lindenbaum J. Small intestine dysfunction in Pakistanis and Americans resident in Pakistan. Am J Clin Nutr. 1968 Sep;21(9):1023–1029. doi: 10.1093/ajcn/21.9.1023. [DOI] [PubMed] [Google Scholar]
- Menzies I. S., Mount J. N., Wheeler M. J. Quantitative estimation of clinically important monosaccharides in plasma by rapid thin layer chromatography. Ann Clin Biochem. 1978 Mar;15(2):65–76. doi: 10.1177/000456327801500116. [DOI] [PubMed] [Google Scholar]
- Menzies I. S. Quantitative estimation of sugars in blood and urine by paper chromatography using direct densitometry. J Chromatogr. 1973 Jun 27;81(1):109–127. doi: 10.1016/s0021-9673(01)82322-0. [DOI] [PubMed] [Google Scholar]
- Noone C., Menzies I. S., Banatvala J. E., Scopes J. W. Intestinal permeability and lactose hydrolysis in human rotaviral gastroenteritis assessed simultaneously by non-invasive differential sugar permeation. Eur J Clin Invest. 1986 Jun;16(3):217–225. doi: 10.1111/j.1365-2362.1986.tb01332.x. [DOI] [PubMed] [Google Scholar]
- Peled Y., Watz C., Gilat T. Measurement of intestinal permeability using 51Cr-EDTA. Am J Gastroenterol. 1985 Oct;80(10):770–773. [PubMed] [Google Scholar]
- SPRINZ H., SRIBHIBHADH R., GANGAROSA E. J., BENYAJATI C., KUNDEL D., HALSTEAD S. Biopsy of small bowel of Thai people. With special reference to recovery from Asiatic cholera and to an intestinal malabsorption syndrome. Am J Clin Pathol. 1962 Jul;38:43–51. doi: 10.1093/ajcp/38.1.43. [DOI] [PubMed] [Google Scholar]
- Schenk E. A., Klipstein F. A., Tomasini J. T. Morphologic characteristics of jejunal biopsies from asymptomatic Haitians and Puerto Ricans. Am J Clin Nutr. 1972 Oct;25(10):1080–1083. doi: 10.1093/ajcn/25.10.1080. [DOI] [PubMed] [Google Scholar]
- Sheehy T. W., Legters L. J., Wallace D. K. Tropical jejunitis in Americans serving in Vietnam. Am J Clin Nutr. 1968 Sep;21(9):1013–1022. doi: 10.1093/ajcn/21.9.1013. [DOI] [PubMed] [Google Scholar]
- Steffen R. Epidemiologic studies of travelers' diarrhea, severe gastrointestinal infections, and cholera. Rev Infect Dis. 1986 May-Jun;8 (Suppl 2):S122–S130. doi: 10.1093/clinids/8.supplement_2.s122. [DOI] [PubMed] [Google Scholar]
- Travis S., Menzies I. Intestinal permeability: functional assessment and significance. Clin Sci (Lond) 1992 May;82(5):471–488. doi: 10.1042/cs0820471. [DOI] [PubMed] [Google Scholar]
- Ukabam S. O., Homeida M. M., Cooper B. T. Small intestinal permeability in normal Sudanese subjects: evidence of tropical enteropathy. Trans R Soc Trop Med Hyg. 1986;80(2):204–207. doi: 10.1016/0035-9203(86)90010-6. [DOI] [PubMed] [Google Scholar]
- Weaver L. T., Chapman P. D., Madeley C. R., Laker M. F., Nelson R. Intestinal permeability changes and excretion of micro-organisms in stools of infants with diarrhoea and vomiting. Arch Dis Child. 1985 Apr;60(4):326–332. doi: 10.1136/adc.60.4.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood G. M., Gearty J. C., Cooper B. T. Small bowel morphology in British Indian and Afro-Caribbean subjects: evidence of tropical enteropathy. Gut. 1991 Mar;32(3):256–259. doi: 10.1136/gut.32.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. J., Watts M. T., Bhatt B. D., Ho H. Intestinal permeability to [51Cr]EDTA in infectious diarrhea. Dig Dis Sci. 1993 Sep;38(9):1651–1657. doi: 10.1007/BF01303174. [DOI] [PubMed] [Google Scholar]