Abstract
BACKGROUND—The polymorphism of apolipoprotein E has been suggested to be associated with the cholesterol content of gallstones, the crystallisation rate of gall bladder bile, and the prevalence of gallstone disease (GSD). AIMS—To investigate whether apolipoprotein E polymorphism modulates the susceptibility to GSD at the population level and to study the possible associations between impaired glucose tolerance, diabetes, and GSD. METHODS—Apolipoprotein E phenotypes were determined in a middle aged cohort of 261 randomly selected hypertensive men, 259 control men, 257 hypertensive women, and 267 control women. All subjects without a documented history of diabetes were submitted to a two hour oral glucose tolerance test (OGTT). GSD was verified by ultrasonography. RESULTS—In women with apolipoprotein E2 (phenotypes E2/2, 2/3, and 2/4) compared with women without E2 (E3/3, 4/3, and 4/4), the odds ratio for GSD was 0.28 (95% confidence interval 0.08-0.92). There was no protective effect in men. The relative risk for GSD was 1.2 (0.8-1.7) for hypertensive women and 1.8(1.0-2.7) for hypertensive men. In a stepwise multiple logistic regression model, E2 protected against GSD in women, whereas two hour blood glucose in the OGTT, serum insulin, and plasma triglycerides were risk factors. Elevated blood glucose during the OGTT was also a significant risk factor for GSD in men. CONCLUSIONS—The data suggest that apolipoprotein E2 is a genetic factor providing protection against GSD in women. In contrast, impaired glucose tolerance and frank diabetes are associated with the risk of GSD.
Keywords: apolipoprotein E; gallstone disease; diabetes; impaired glucose tolerance; cholesterol
Full Text
The Full Text of this article is available as a PDF (131.0 KB).
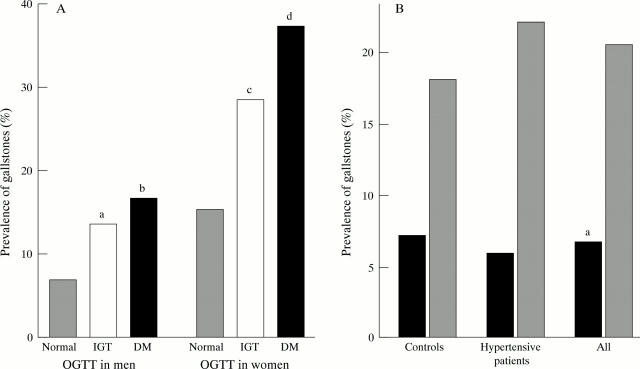
Figure 1 .
(A) Prevalence of gallstone disease in normoglycaemic subjects, subjects with impaired glucose tolerance (IGT), and diabetes mellitus (DM). For (a) OR=2.08 (95% CI 0.99-4.40); (b) OR=2.65 (1.17-5.99); (c) OR=2.20 (1.29-3.77); and (d) OR=3.29 (1.67-6.47) compared with normoglycaemic subjects. (B) Prevalence of gallstone disease in control, hypertensive, and all women with at least one ε2 allele (phenotypes E2/2, 2/3, and 2/4; black bars) and without the ε2 allele (phenotypes E3/3, 4/3, and 4/4; shaded bars). For (a) OR=0.28 (95% CI 0.08-0.92).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antero Kesäniemi Y., Koskenvuo M., Vuoristo M., Miettinen T. A. Biliary lipid composition in monozygotic and dizygotic pairs of twins. Gut. 1989 Dec;30(12):1750–1756. doi: 10.1136/gut.30.12.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attili A. F., Capocaccia R., Carulli N., Festi D., Roda E., Barbara L., Capocaccia L., Menotti A., Okolicsanyi L., Ricci G. Factors associated with gallstone disease in the MICOL experience. Multicenter Italian Study on Epidemiology of Cholelithiasis. Hepatology. 1997 Oct;26(4):809–818. doi: 10.1002/hep.510260401. [DOI] [PubMed] [Google Scholar]
- Bennion L. J., Grundy S. M. Risk factors for the development of cholelithiasis in man (second of two parts). N Engl J Med. 1978 Nov 30;299(22):1221–1227. doi: 10.1056/NEJM197811302992205. [DOI] [PubMed] [Google Scholar]
- Bertomeu A., Ros E., Zambón D., Vela M., Pérez-Ayuso R. M., Targarona E., Trías M., Sanllehy C., Casals E., Ribó J. M. Apolipoprotein E polymorphism and gallstones. Gastroenterology. 1996 Dec;111(6):1603–1610. doi: 10.1016/s0016-5085(96)70023-9. [DOI] [PubMed] [Google Scholar]
- Busch N., Matern S. Current concepts in cholesterol gallstone pathogenesis. Eur J Clin Invest. 1991 Oct;21(5):453–460. doi: 10.1111/j.1365-2362.1991.tb01394.x. [DOI] [PubMed] [Google Scholar]
- Danzinger R. G., Gordon H., Schoenfield L. J., Thistle J. L. Lithogenic bile in siblings of young women with cholelithiasis. Mayo Clin Proc. 1972 Oct;47(10):762–766. [PubMed] [Google Scholar]
- Davignon J., Gregg R. E., Sing C. F. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988 Jan-Feb;8(1):1–21. doi: 10.1161/01.atv.8.1.1. [DOI] [PubMed] [Google Scholar]
- De Santis A., Attili A. F., Ginanni Corradini S., Scafato E., Cantagalli A., De Luca C., Pinto G., Lisi D., Capocaccia L. Gallstones and diabetes: a case-control study in a free-living population sample. Hepatology. 1997 Apr;25(4):787–790. doi: 10.1002/hep.510250401. [DOI] [PubMed] [Google Scholar]
- Diehl A. K., Haffner S. M., Hazuda H. P., Stern M. P. Coronary risk factors and clinical gallbladder disease: an approach to the prevention of gallstones? Am J Public Health. 1987 Jul;77(7):841–845. doi: 10.2105/ajph.77.7.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehnholm C., Lukka M., Kuusi T., Nikkilä E., Utermann G. Apolipoprotein E polymorphism in the Finnish population: gene frequencies and relation to lipoprotein concentrations. J Lipid Res. 1986 Mar;27(3):227–235. [PubMed] [Google Scholar]
- Grundy S. M., Metzger A. L., Adler R. D. Mechanisms of lithogenic bile formation in American Indian women with cholesterol gallstones. J Clin Invest. 1972 Dec;51(12):3026–3043. doi: 10.1172/JCI107130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré L. H. The lack of a positive association between symptomatic cholesterol cholelithiasis and clinical diabetes mellitus: a retrospective study. J Chronic Dis. 1980;33(8):465–469. doi: 10.1016/0021-9681(80)90071-5. [DOI] [PubMed] [Google Scholar]
- Johnston D. E., Kaplan M. M. Pathogenesis and treatment of gallstones. N Engl J Med. 1993 Feb 11;328(6):412–421. doi: 10.1056/NEJM199302113280608. [DOI] [PubMed] [Google Scholar]
- Juvonen T., Kervinen K., Kairaluoma M. I., Lajunen L. H., Kesäniemi Y. A. Gallstone cholesterol content is related to apolipoprotein E polymorphism. Gastroenterology. 1993 Jun;104(6):1806–1813. doi: 10.1016/0016-5085(93)90662-v. [DOI] [PubMed] [Google Scholar]
- Juvonen T., Savolainen M. J., Kairaluoma M. I., Lajunen L. H., Humphries S. E., Kesäniemi Y. A. Polymorphisms at the apoB, apoA-I, and cholesteryl ester transfer protein gene loci in patients with gallbladder disease. J Lipid Res. 1995 Apr;36(4):804–812. [PubMed] [Google Scholar]
- Kervinen K., Savolainen M. J., Salokannel J., Hynninen A., Heikkinen J., Ehnholm C., Koistinen M. J., Kesäniemi Y. A. Apolipoprotein E and B polymorphisms--longevity factors assessed in nonagenarians. Atherosclerosis. 1994 Jan;105(1):89–95. doi: 10.1016/0021-9150(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Kervinen K., Södervik H., Mäkelä J., Lehtola J., Niemi M., Kairaluoma M. I., Kesäniemi Y. A. Is the development of adenoma and carcinoma in proximal colon related to apolipoprotein E phenotype? Gastroenterology. 1996 Jun;110(6):1785–1790. doi: 10.1053/gast.1996.v110.pm8964404. [DOI] [PubMed] [Google Scholar]
- Kesäniemi Y. A., Ehnholm C., Miettinen T. A. Intestinal cholesterol absorption efficiency in man is related to apoprotein E phenotype. J Clin Invest. 1987 Aug;80(2):578–581. doi: 10.1172/JCI113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusisto J., Koivisto K., Kervinen K., Mykkänen L., Helkala E. L., Vanhanen M., Hänninen T., Pyörälä K., Kesäniemi Y. A., Riekkinen P. Association of apolipoprotein E phenotypes with late onset Alzheimer's disease: population based study. BMJ. 1994 Sep 10;309(6955):636–638. doi: 10.1136/bmj.309.6955.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liinamaa M. J., Kervinen K., Hannuksela M. L., Kesäniemi Y. A., Savolainen M. J. Effect of apolipoprotein E phenotype on plasma lipids and lipoproteins in alcohol abusers. Alcohol Clin Exp Res. 1997 Jun;21(4):606–612. [PubMed] [Google Scholar]
- Ma J., Yee A., Brewer H. B., Jr, Das S., Potter H. Amyloid-associated proteins alpha 1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer beta-protein into filaments. Nature. 1994 Nov 3;372(6501):92–94. doi: 10.1038/372092a0. [DOI] [PubMed] [Google Scholar]
- Maclure K. M., Hayes K. C., Colditz G. A., Stampfer M. J., Speizer F. E., Willett W. C. Weight, diet, and the risk of symptomatic gallstones in middle-aged women. N Engl J Med. 1989 Aug 31;321(9):563–569. doi: 10.1056/NEJM198908313210902. [DOI] [PubMed] [Google Scholar]
- Muhrbeck O., Ahlberg J. Prevalence of gallstone disease in a Swedish population. Scand J Gastroenterol. 1995 Nov;30(11):1125–1128. doi: 10.3109/00365529509101618. [DOI] [PubMed] [Google Scholar]
- Petitti D. B., Friedman G. D., Klatsky A. L. Association of a history of gallbladder disease with a reduced concentration of high-density-lipoprotein cholesterol. N Engl J Med. 1981 Jun 4;304(23):1396–1398. doi: 10.1056/NEJM198106043042305. [DOI] [PubMed] [Google Scholar]
- Poirier J., Davignon J., Bouthillier D., Kogan S., Bertrand P., Gauthier S. Apolipoprotein E polymorphism and Alzheimer's disease. Lancet. 1993 Sep 18;342(8873):697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- Portincasa P., van Erpecum K. J., van De Meeberg P. C., Dallinga-Thie G. M., de Bruin T. W., van Berge-Henegouwen G. P. Apolipoprotein E4 genotype and gallbladder motility influence speed of gallstone clearance and risk of recurrence after extracorporeal shock-wave lithotripsy. Hepatology. 1996 Sep;24(3):580–587. doi: 10.1002/hep.510240320. [DOI] [PubMed] [Google Scholar]
- Rantala A. O., Päivänsalo M., Kauma H., Lilja M., Savolainen M. J., Reunanen A., Kesäniemi Y. A. Hyperinsulinemia and carotid atherosclerosis in hypertensive and control subjects. Diabetes Care. 1998 Jul;21(7):1188–1193. doi: 10.2337/diacare.21.7.1188. [DOI] [PubMed] [Google Scholar]
- Scragg R. K., Calvert G. D., Oliver J. R. Plasma lipids and insulin in gall stone disease: a case-control study. Br Med J (Clin Res Ed) 1984 Sep 1;289(6444):521–525. doi: 10.1136/bmj.289.6444.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terkeltaub R. A., Dyer C. A., Martin J., Curtiss L. K. Apolipoprotein (apo) E inhibits the capacity of monosodium urate crystals to stimulate neutrophils. Characterization of intraarticular apo E and demonstration of apo E binding to urate crystals in vivo. J Clin Invest. 1991 Jan;87(1):20–26. doi: 10.1172/JCI114971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijs C., Knipschild P., Brombacher P. Serum lipids and gallstones: a case-control study. Gastroenterology. 1990 Sep;99(3):843–849. doi: 10.1016/0016-5085(90)90978-a. [DOI] [PubMed] [Google Scholar]
- Utermann G. Apolipoprotein E polymorphism in health and disease. Am Heart J. 1987 Feb;113(2 Pt 2):433–440. doi: 10.1016/0002-8703(87)90610-7. [DOI] [PubMed] [Google Scholar]
- Van Erpecum K. J., Carey M. C. Apolipoprotein E4: another risk factor for cholesterol gallstone formation? Gastroenterology. 1996 Dec;111(6):1764–1767. doi: 10.1016/s0016-5085(96)70044-6. [DOI] [PubMed] [Google Scholar]
- Vlahcevic Z. R., Bell C. C., Jr, Buhac I., Farrar J. T., Swell L. Diminished bile acid pool size in patients with gallstones. Gastroenterology. 1970 Aug;59(2):165–173. [PubMed] [Google Scholar]