Abstract
BACKGROUND AND AIMS—Laser induced fluorescence (LIF) from colonic mucosa was measured in vivo with and without δ aminolevulinic acid (ALA) in an attempt to differentiate between neoplasia and non-neoplasia in real time during colonoscopy. METHODS—Spectra from 32 adenomas, 68 normal sites, and 14 hyperplastic polyps in 41 patients were obtained with a point monitoring system. Twenty one of the patients had been given a low dose of ALA as a photosensitiser before the examination. Light of 337, 405, or 436 nm wavelength was used as excitation. Stepwise multivariate linear regression analysis was performed. RESULTS—With 337 nm excitation, 100% sensitivity and 96% specificity was obtained between normal mucosa and adenomas. Seventy seven per cent of the hyperplastic polyps were classified as non-neoplastic. When exciting with 405 and 436 nm, the possibility of distinguishing different types of tissue was considerably better in the ALA patients than in the non-ALA patients. CONCLUSIONS—The in vivo point measurements imply that a good discrimination between normal tissue and adenomatous polyps can be obtained using the LIF technique. Excitation at 337 nm and at 405 nm or 436 nm using ALA gives good results. LIF also shows potential for distinguishing adenomatous from hyperplastic polyps. The number of detection wavelengths could be reduced if chosen properly.
Keywords: fluorescence; diagnostics; adenoma; colon; laser
Full Text
The Full Text of this article is available as a PDF (173.0 KB).
Figure 1 .
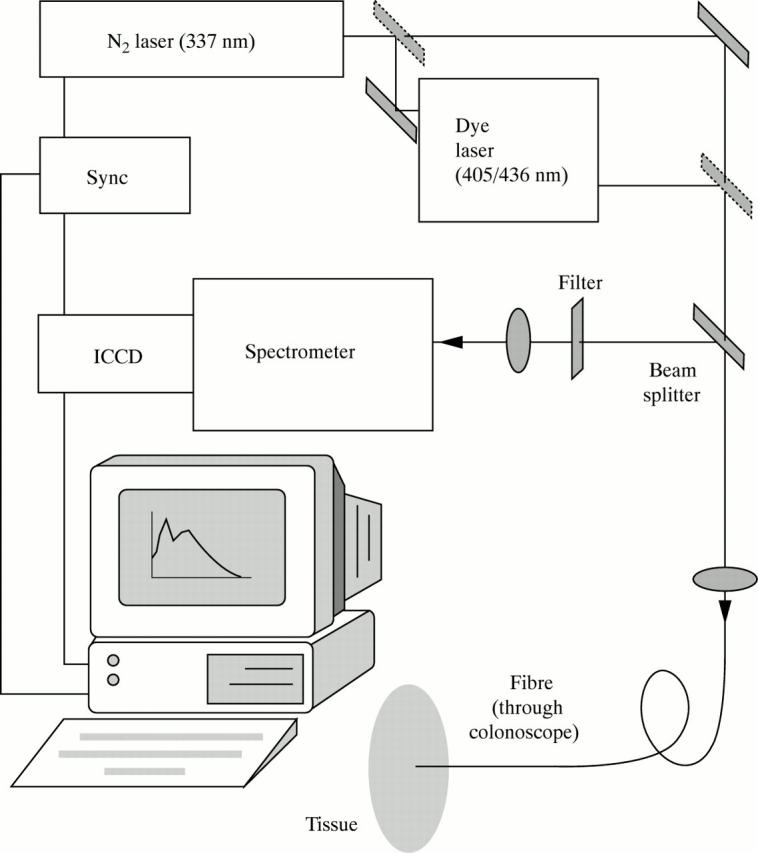
Experimental set up for in vivo LIF recording.
Figure 2 .
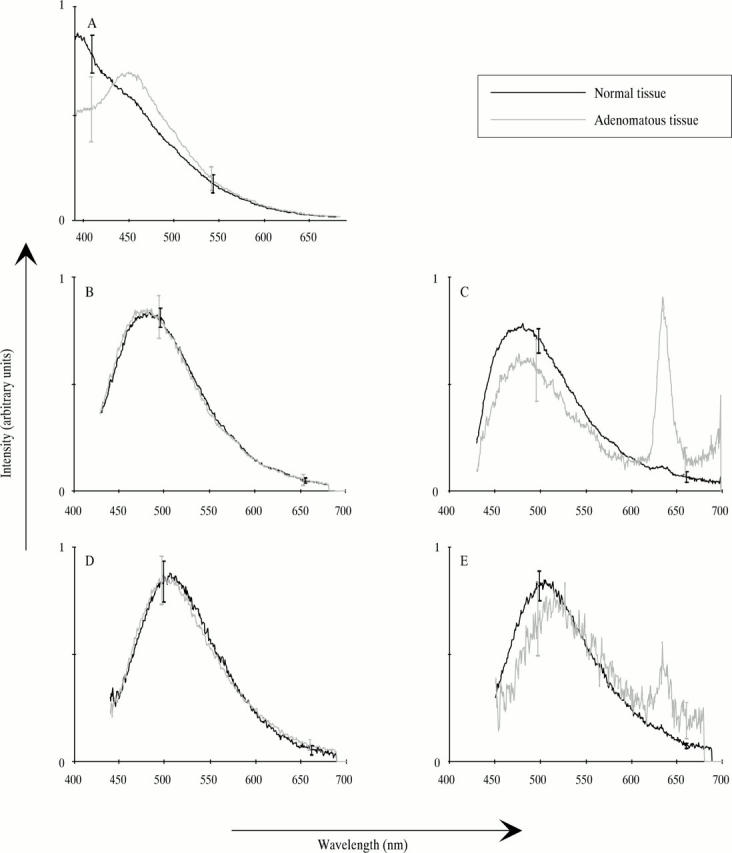
Typical in vivo spectra from normal and adenomatous colon tissue. The spectra are normalised to a total intensity of 1. Error bars represent standard deviations. (A) 337 nm excitation, both ALA and non-ALA patients; (B) 405 nm excitation, non-ALA patients; (C) 405 nm excitation, ALA patients; (D) 436 nm excitation, non-ALA patients; (E) 436 nm excitation, ALA patients.
Figure 3 .
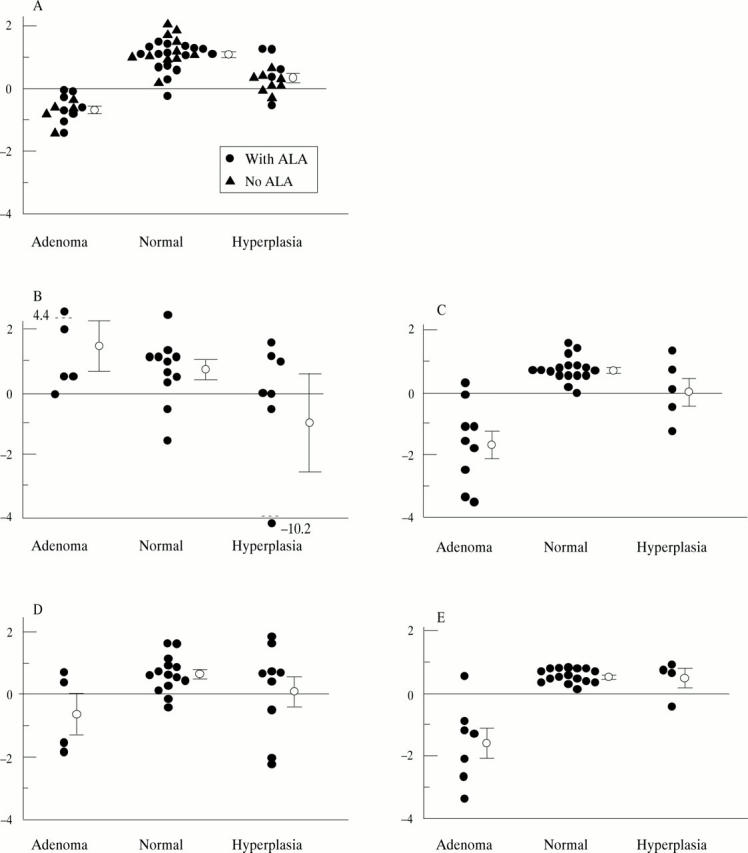
Fluorescence score derived using stepwise MVLR analysis. The circles with error bars represent mean scores with standard errors. (A) 337 nm excitation—evaluation at 409, 440, 498, 572, and 661 nm; (B) 405 nm excitation, non-ALA patients—evaluation at 498, 543, 560, 590, 602, and 661 nm; (C) 405 nm excitation, ALA patients—evaluation at 498, 543, 560, 572, 602, and 661 nm; (D) 436 nm excitation, non-ALA patients—evaluation at 498, 543, 560, 572, 590, and 602 nm; (E) 436 nm excitation, ALA patients—evaluation at 498 and 661 nm.
Figure 4 .
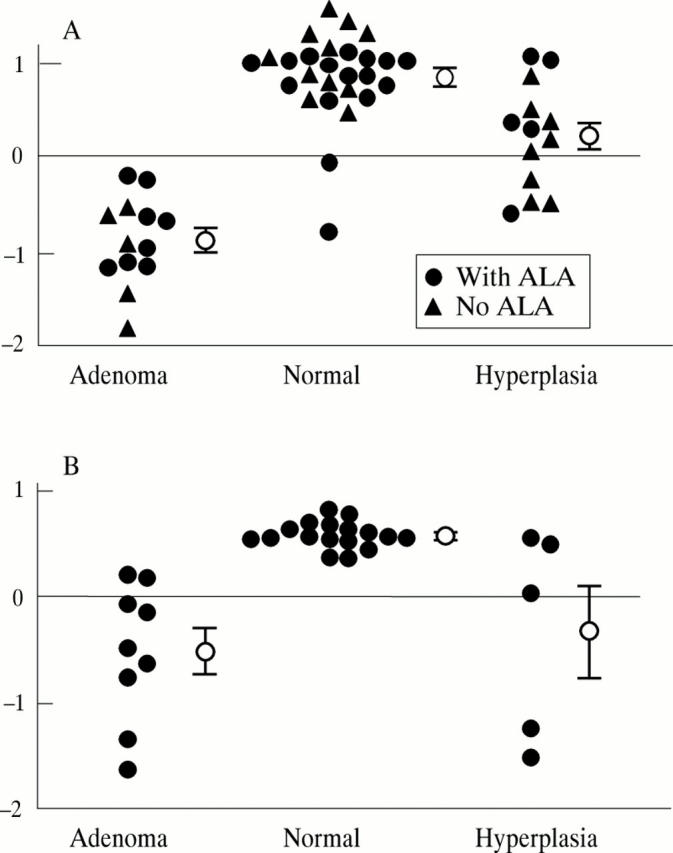
MVLR results using two evaluation wavelengths. The circles with error bars represent mean scores with standard errors. (A) 337 nm excitation, evaluation at 409 and 543 nm; (B) 405 nm excitation, ALA patients, evaluation at 498 and 661 nm.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baert L., Berg R., Van Damme B., D'Hallewin M. A., Johansson J., Svanberg K., Svanberg S. Clinical fluorescence diagnosis of human bladder carcinoma following low-dose Photofrin injection. Urology. 1993 Apr;41(4):322–330. doi: 10.1016/0090-4295(93)90588-2. [DOI] [PubMed] [Google Scholar]
- Cothren R. M., Richards-Kortum R., Sivak M. V., Jr, Fitzmaurice M., Rava R. P., Boyce G. A., Doxtader M., Blackman R., Ivanc T. B., Hayes G. B. Gastrointestinal tissue diagnosis by laser-induced fluorescence spectroscopy at endoscopy. Gastrointest Endosc. 1990 Mar-Apr;36(2):105–111. doi: 10.1016/s0016-5107(90)70961-3. [DOI] [PubMed] [Google Scholar]
- Dailey H. A., Smith A. Differential interaction of porphyrins used in photoradiation therapy with ferrochelatase. Biochem J. 1984 Oct 15;223(2):441–445. doi: 10.1042/bj2230441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyerdahl H., Wang I., Liu D. L., Berg R., Andersson-Engels S., Peng Q., Moan J., Svanberg S., Svanberg K. Pharmacokinetic studies on 5-aminolevulinic acid-induced protoporphyrin IX accumulation in tumours and normal tissues. Cancer Lett. 1997 Jan 30;112(2):225–231. doi: 10.1016/s0304-3835(96)04576-4. [DOI] [PubMed] [Google Scholar]
- Kapadia C. R., Cutruzzola F. W., O'Brien K. M., Stetz M. L., Enriquez R., Deckelbaum L. I. Laser-induced fluorescence spectroscopy of human colonic mucosa. Detection of adenomatous transformation. Gastroenterology. 1990 Jul;99(1):150–157. doi: 10.1016/0016-5085(90)91242-x. [DOI] [PubMed] [Google Scholar]
- Kennedy J. C., Pottier R. H. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B. 1992 Jul 30;14(4):275–292. doi: 10.1016/1011-1344(92)85108-7. [DOI] [PubMed] [Google Scholar]
- Kennedy J. C., Pottier R. H., Pross D. C. Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience. J Photochem Photobiol B. 1990 Jun;6(1-2):143–148. doi: 10.1016/1011-1344(90)85083-9. [DOI] [PubMed] [Google Scholar]
- Leibovici L., Schoenfeld N., Yehoshua H. A., Mamet R., Rakowsky E., Shindel A., Atsmon A. Activity of porphobilinogen deaminase in peripheral blood mononuclear cells of patients with metastatic cancer. Cancer. 1988 Dec 1;62(11):2297–2300. doi: 10.1002/1097-0142(19881201)62:11<2297::aid-cncr2820621106>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Loh C. S., MacRobert A. J., Bedwell J., Regula J., Krasner N., Bown S. G. Oral versus intravenous administration of 5-aminolaevulinic acid for photodynamic therapy. Br J Cancer. 1993 Jul;68(1):41–51. doi: 10.1038/bjc.1993.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesini R., Brambilla M., Pignoli E., Bottiroli G., Croce A. C., Dal Fante M., Spinelli P., di Palma S. Light-induced fluorescence spectroscopy of adenomas, adenocarcinomas and non-neoplastic mucosa in human colon. I. In vitro measurements. J Photochem Photobiol B. 1992 Jul 15;14(3):219–230. doi: 10.1016/1011-1344(92)85100-9. [DOI] [PubMed] [Google Scholar]
- O'Brien K. M., Gmitro A. F., Gindi G. R., Stetz M. L., Cutruzzola F. W., Laifer L. I., Deckelbaum L. I. Development and evaluation of spectral classification algorithms for fluorescence guided laser angioplasty. IEEE Trans Biomed Eng. 1989 Apr;36(4):424–431. doi: 10.1109/10.18748. [DOI] [PubMed] [Google Scholar]
- Peng Q., Berg K., Moan J., Kongshaug M., Nesland J. M. 5-Aminolevulinic acid-based photodynamic therapy: principles and experimental research. Photochem Photobiol. 1997 Feb;65(2):235–251. doi: 10.1111/j.1751-1097.1997.tb08549.x. [DOI] [PubMed] [Google Scholar]
- Peng Q., Warloe T., Berg K., Moan J., Kongshaug M., Giercksky K. E., Nesland J. M. 5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer. 1997 Jun 15;79(12):2282–2308. doi: 10.1002/(sici)1097-0142(19970615)79:12<2282::aid-cncr2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Richards-Kortum R., Rava R. P., Petras R. E., Fitzmaurice M., Sivak M., Feld M. S. Spectroscopic diagnosis of colonic dysplasia. Photochem Photobiol. 1991 Jun;53(6):777–786. doi: 10.1111/j.1751-1097.1991.tb09892.x. [DOI] [PubMed] [Google Scholar]
- Schoenfeld N., Mamet R., Nordenberg Y., Shafran M., Babushkin T., Malik Z. Protoporphyrin biosynthesis in melanoma B16 cells stimulated by 5-aminolevulinic acid and chemical inducers: characterization of photodynamic inactivation. Int J Cancer. 1994 Jan 2;56(1):106–112. doi: 10.1002/ijc.2910560119. [DOI] [PubMed] [Google Scholar]
- Schomacker K. T., Frisoli J. K., Compton C. C., Flotte T. J., Richter J. M., Deutsch T. F., Nishioka N. S. Ultraviolet laser-induced fluorescence of colonic polyps. Gastroenterology. 1992 Apr;102(4 Pt 1):1155–1160. [PubMed] [Google Scholar]
- Schomacker K. T., Frisoli J. K., Compton C. C., Flotte T. J., Richter J. M., Nishioka N. S., Deutsch T. F. Ultraviolet laser-induced fluorescence of colonic tissue: basic biology and diagnostic potential. Lasers Surg Med. 1992;12(1):63–78. doi: 10.1002/lsm.1900120111. [DOI] [PubMed] [Google Scholar]
- Svaasand L. O., Wyss P., Wyss M. T., Tadir Y., Tromberg B. J., Berns M. W. Dosimetry model for photodynamic therapy with topically administered photosensitizers. Lasers Surg Med. 1996;18(2):139–149. doi: 10.1002/(SICI)1096-9101(1996)18:2<139::AID-LSM3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Svanberg K., Andersson T., Killander D., Wang I., Stenram U., Andersson-Engels S., Berg R., Johansson J., Svanberg S. Photodynamic therapy of non-melanoma malignant tumours of the skin using topical delta-amino levulinic acid sensitization and laser irradiation. Br J Dermatol. 1994 Jun;130(6):743–751. doi: 10.1111/j.1365-2133.1994.tb03412.x. [DOI] [PubMed] [Google Scholar]
- von Rueden D. G., McBrearty F. X., Clements B. M., Woratyla S. Photo detection of carcinoma of the colon in a rat model: a pilot study. J Surg Oncol. 1993 May;53(1):43–46. doi: 10.1002/jso.2930530112. [DOI] [PubMed] [Google Scholar]


