Abstract
BACKGROUND—Helicobacter pylori affects gastric epithelium integrity by acceleration of apoptosis. However, it remains unclear what product of the bacteria causes apoptosis, or whether or not the apoptosis is involved in the development of ulcers. AIMS—To elucidate the factor from H pylori that causes acceleration of apoptosis and the role of apoptosis in the development of duodenal ulcer in H pylori infection. PATIENTS—Five H pylori negative healthy volunteers, 47 H pylori positive patients with duodenal ulcer, and 35 H pylori positive patients with gastric ulcer. METHODS—An endoscopic examination was carried out to diagnose ulcers and determine their clinical stage. To analyse apoptosis, a cell cycle analysis was performed using biopsy specimens. RESULTS—There was a significant correlation between the urease activity of the H pylori strain and the level of apoptosis induced by this bacterial strain. Moreover, in duodenal ulcer patients infected with H pylori, the patients with an active ulcer exhibited a significantly higher level of apoptosis than those with ulcers at both the healing and scarring stages. CONCLUSION—These findings suggest that acceleration of apoptosis in the antral mucosa caused by the urease of H pylori plays a crucial role in the development of ulcers in the duodenum.
Keywords: apoptosis; Helicobacter pylori; urease; duodenal ulcer; gastric ulcer
Full Text
The Full Text of this article is available as a PDF (160.2 KB).
Figure 1 .
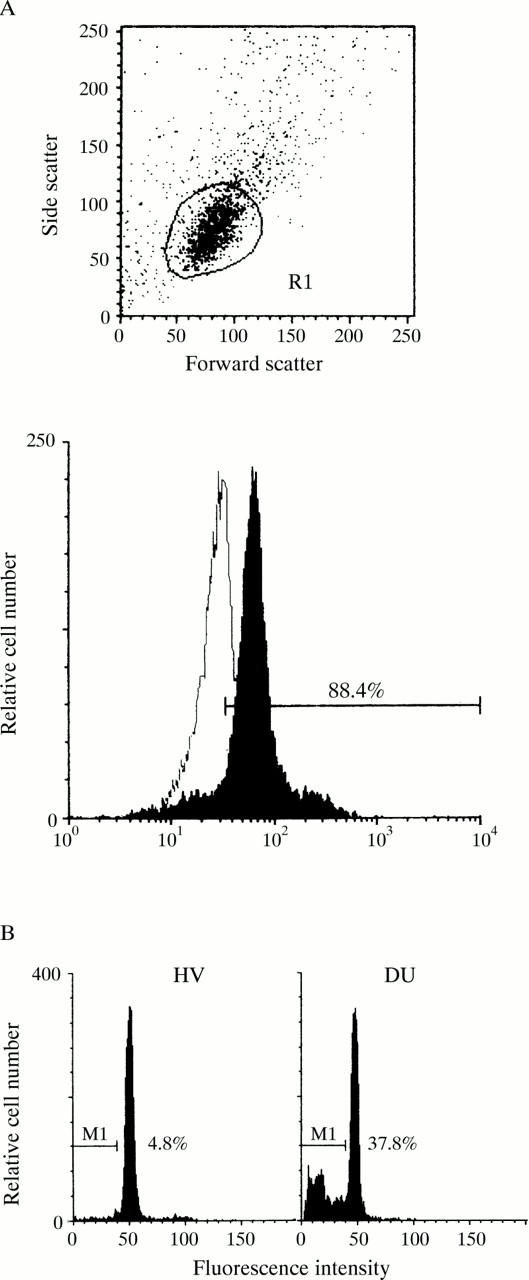
Flow cytometry analysis of gastric mucosal cells. (A) The flow cytometer was gated to include single cells but to exclude any cell debris and clumps of cells according to the forward and side scatter patterns (upper panel). Single gastric mucosal cells were stained with mouse anti-epidermal growth factor receptor antibody and fluorescein isothiocyanate conjugated anti-mouse IgG antibody (lower panel, closed configuration) or fluorescein isothiocyanate conjugated anti-mouse IgG antibody only (lower panel, open configuration). (B) Representative data of the cell cycle analysis using specimens from healthy volunteers (HV) and patients with duodenal ulcer (DU) are shown, and the percentage of apoptosis cells in these specimens is also indicated.
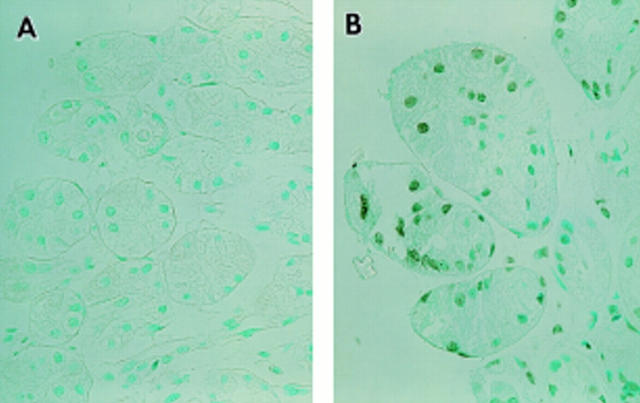
Figure 2 .
Gastric antral biopsy samples obtained from a healthy volunteer (A) and a patient with a duodenal ulcer (B) were examined by TUNEL histochemistry.
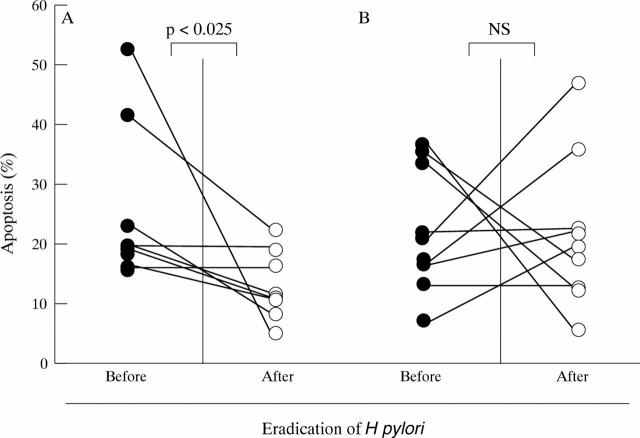
Figure 3 .
Effect of the eradication of H pylori on the level of apoptosis. Gastric biopsy samples were taken from (A) patients with DU and (B) those with GU before the administration of antibiotics and three months after eradication therapy. The level of apoptosis in these samples was evaluated by flow cytometric analysis. A statistical analysis was performed using the paired t test.
Figure 4 .

Relation between apoptosis and the propeties of H pylori. The relation between the level of apoptosis in mucosal cells examined by flow cytometry and the bacteriological properties of H pylori including the number of bacteria in the mucosal tissue (A), the activity of the vacuolating toxin (VT) in the culture supernatant of bacteria (B), and the urease activity of the bacteria (C) were plotted. The relations between these factors were then statistically analysed by a scattergram plot.
Figure 5 .
Level of apoptosis at each clinical stage in ulcer disease. The level of apoptosis was examined by flow cytometry using gastric biopsy samples obtained from healthy volunteers and patients with duodenal ulcer at the active, healing, or scarring stage, and patients with gastric ulcer at the active, healing, or scarring stage. Statistical analysis was by Student's t test.
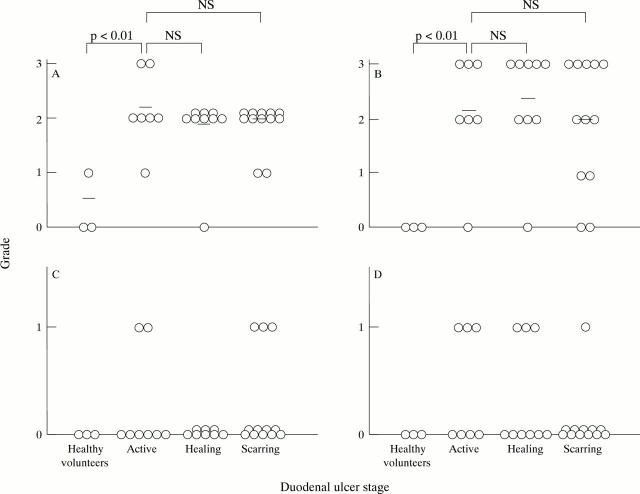
Figure 6 .
Histological analysis of gastric biopsy specimens. Histological features including lymphocyte infiltration (A), neutrophil infiltration (B), intestinal metaplasia (C), and epithelial erosion (D) were examined using gastric biopsy samples from healthy volunteers and patients with active, healing, and scarring duodenal ulcers, and their grades determined. Statistical analysis was by the Mann-Whitney U test.
Figure 7 .

Number of H pylori in the antral mucosa of patients with duodenal ulcer at different clinical stages. Antral biopsy samples were taken from patients with active, healing, and scarring ulcers, and the number of H pylori colonising the sample was determined.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton J. C., Peek R. M., Jr, Tham K. T., Cover T. L., Blaser M. J. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology. 1997 Jan;112(1):92–99. doi: 10.1016/s0016-5085(97)70223-3. [DOI] [PubMed] [Google Scholar]
- Chittajallu R. S., Neithercut W. D., Macdonald A. M., McColl K. E. Effect of increasing Helicobacter pylori ammonia production by urea infusion on plasma gastrin concentrations. Gut. 1991 Jan;32(1):21–24. doi: 10.1136/gut.32.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. Y., Opekun A., Lew G. M., Klein P. D., Walsh J. H. Helicobacter pylori-associated exaggerated gastrin release in duodenal ulcer patients. The effect of bombesin infusion and urea ingestion. Gastroenterology. 1991 Jun;100(6):1571–1575. doi: 10.1016/0016-5085(91)90654-4. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Coates P. J., Ansari B., Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994 Dec;107(Pt 12):3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- Kabir A. M., Aiba Y., Takagi A., Kamiya S., Miwa T., Koga Y. Prevention of Helicobacter pylori infection by lactobacilli in a gnotobiotic murine model. Gut. 1997 Jul;41(1):49–55. doi: 10.1136/gut.41.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya S., Osaki T., Kumada J., Yamaguchi H., Taguchi H. Effect of sofalcone on adherence, production of vacuolating toxin, and induction of interleukin-8 secretion by Helicobacter pylori. J Clin Gastroenterol. 1997;25 (Suppl 1):S172–S178. doi: 10.1097/00004836-199700001-00028. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Okazaki K., Murakami K. Adhesion of Helicobacter pylori to gastric epithelial cells in primary cultures obtained from stomachs of various animals. Infect Immun. 1993 Oct;61(10):4058–4063. doi: 10.1128/iai.61.10.4058-4063.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Fox J., Hazell S. Pathogenicity of Helicobacter pylori: a perspective. Infect Immun. 1993 May;61(5):1601–1610. doi: 10.1128/iai.61.5.1601-1610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S., Beardshall K., Haddad G., Playford R., Ghosh P., Calam J. Campylobacter pylori and duodenal ulcers: the gastrin link. Lancet. 1989 May 27;1(8648):1167–1168. doi: 10.1016/s0140-6736(89)92752-9. [DOI] [PubMed] [Google Scholar]
- Lichtenberger L. M., Dial E. J., Romero J. J., Lechago J., Jarboe L. A., Wolfe M. M. Role of luminal ammonia in the development of gastropathy and hypergastrinemia in the rat. Gastroenterology. 1995 Feb;108(2):320–329. doi: 10.1016/0016-5085(95)90056-x. [DOI] [PubMed] [Google Scholar]
- Marshall B. J. Helicobacter pylori. Am J Gastroenterol. 1994 Aug;89(8 Suppl):S116–S128. [PubMed] [Google Scholar]
- Marshall B. J., Langton S. R. Urea hydrolysis in patients with Campylobacter pyloridis infection. Lancet. 1986 Apr 26;1(8487):965–966. doi: 10.1016/s0140-6736(86)91060-3. [DOI] [PubMed] [Google Scholar]
- Mori T., Ando K., Tanaka K., Ikeda Y., Koga Y. Fas-mediated apoptosis of the hematopoietic progenitor cells in mice infected with murine cytomegalovirus. Blood. 1997 May 15;89(10):3565–3573. [PubMed] [Google Scholar]
- Moss S. F., Calam J., Agarwal B., Wang S., Holt P. R. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996 Apr;38(4):498–501. doi: 10.1136/gut.38.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Saita H., Teramura S., Dekigai H., Asagoe K., Kusaka S., Kita T. Gastric ammonia has a potent ulcerogenic action on the rat stomach. Gastroenterology. 1993 Dec;105(6):1710–1715. doi: 10.1016/0016-5085(93)91067-r. [DOI] [PubMed] [Google Scholar]
- Olbe L., Hamlet A., Dalenbäck J., Fändriks L. A mechanism by which Helicobacter pylori infection of the antrum contributes to the development of duodenal ulcer. Gastroenterology. 1996 May;110(5):1386–1394. doi: 10.1053/gast.1996.v110.pm8613042. [DOI] [PubMed] [Google Scholar]
- Sakita T., Oguro Y., Takasu S., Fukutomi H., Miwa T. Observations on the healing of ulcerations in early gastric cancer. The life cycle of the malignant ulcer. Gastroenterology. 1971 May;60(5):835–passim. [PubMed] [Google Scholar]
- Smoot D. T., Mobley H. L., Chippendale G. R., Lewison J. F., Resau J. H. Helicobacter pylori urease activity is toxic to human gastric epithelial cells. Infect Immun. 1990 Jun;58(6):1992–1994. doi: 10.1128/iai.58.6.1992-1994.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995 Mar 10;267(5203):1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Tsujii M., Kawano S., Tsuji S., Ito T., Nagano K., Sasaki Y., Hayashi N., Fusamoto H., Kamada T. Cell kinetics of mucosal atrophy in rat stomach induced by long-term administration of ammonia. Gastroenterology. 1993 Mar;104(3):796–801. doi: 10.1016/0016-5085(93)91015-a. [DOI] [PubMed] [Google Scholar]
- Wagner S., Beil W., Westermann J., Logan R. P., Bock C. T., Trautwein C., Bleck J. S., Manns M. P. Regulation of gastric epithelial cell growth by Helicobacter pylori: offdence for a major role of apoptosis. Gastroenterology. 1997 Dec;113(6):1836–1847. doi: 10.1016/s0016-5085(97)70003-9. [DOI] [PubMed] [Google Scholar]
- el-Omar E. M., Penman I. D., Ardill J. E., Chittajallu R. S., Howie C., McColl K. E. Helicobacter pylori infection and abnormalities of acid secretion in patients with duodenal ulcer disease. Gastroenterology. 1995 Sep;109(3):681–691. doi: 10.1016/0016-5085(95)90374-7. [DOI] [PubMed] [Google Scholar]