Abstract
BACKGROUND—The pathogenesis of pancreatic fibrosis is unknown. In the liver, stellate cells play a major role in fibrogenesis by synthesising increased amounts of collagen and other extracellular matrix (ECM) proteins when activated by profibrogenic mediators such as cytokines and oxidant stress. AIMS—To determine whether cultured rat pancreatic stellate cells produce collagen and other ECM proteins, and exhibit signs of activation when exposed to the cytokines platelet derived growth factor (PDGF) or transforming growth factor β (TGF-β). METHODS—Cultured pancreatic stellate cells were immunostained for the ECM proteins procollagen III, collagen I, laminin, and fibronectin using specific polyclonal antibodies. For cytokine studies, triplicate wells of cells were incubated with increasing concentrations of PDGF or TGF-β. RESULTS—Cultured pancreatic stellate cells stained strongly positive for all ECM proteins tested. Incubation of cells with 1, 5, and 10 ng/ml PDGF led to a significant dose related increase in cell counts as well as in the incorporation of 3H-thymidine into DNA. Stellate cells exposed to 0.25, 0.5, and 1 ng/ml TGF-β showed a dose dependent increase in α smooth muscle actin expression and increased collagen synthesis. In addition, TGF-β increased the expression of PDGF receptors on stellate cells. CONCLUSIONS—Pancreatic stellate cells produce collagen and other extracellular matrix proteins, and respond to the cytokines PDGF and TGF-β by increased proliferation and increased collagen synthesis. These results suggest an important role for stellate cells in pancreatic fibrogenesis.
Keywords: pancreatic fibrosis; stellate cell activation; cytokines
Full Text
The Full Text of this article is available as a PDF (184.3 KB).
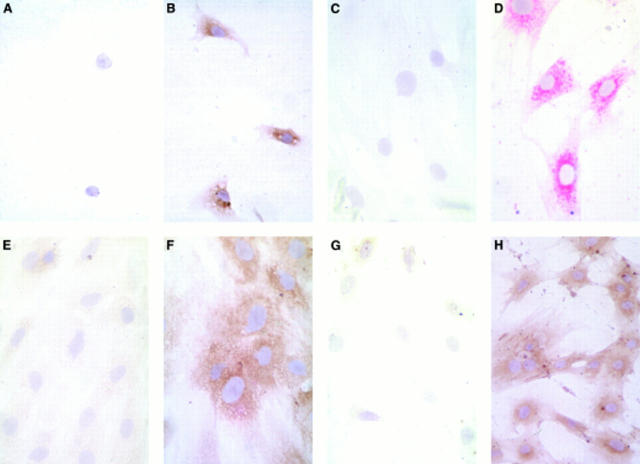
Figure 1 .
Pancreatic stellate cells in culture stained strongly positive for procollagen III, collagen I, laminin, and fibronectin (panels B, D, F, and H respectively) compared with control cells incubated with the appropriate non-immune sera (panels A, C, E, and G respectively). Staining for the collagens and for laminin was perinuclear in distribution; that for fibronectin was diffuse.
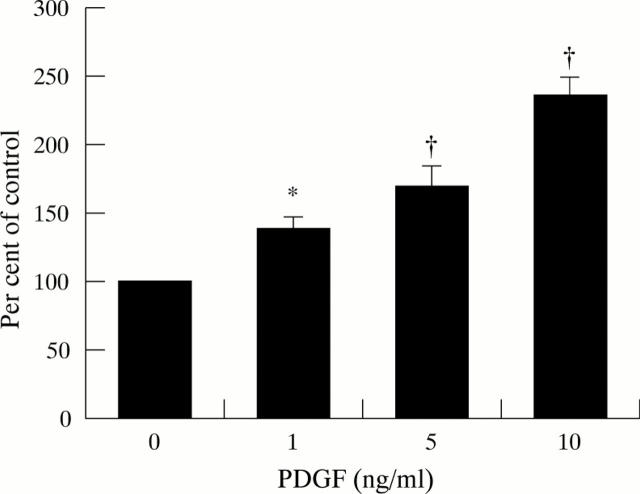
Figure 2 .
Effect of PDGF on cell counts (five separate cell preparations). Results are expressed as a percentage of control values (cells not incubated with PDGF). Increasing concentrations of PDGF resulted in a dose related increase in cell numbers (*p<0.025, †p<0.001).
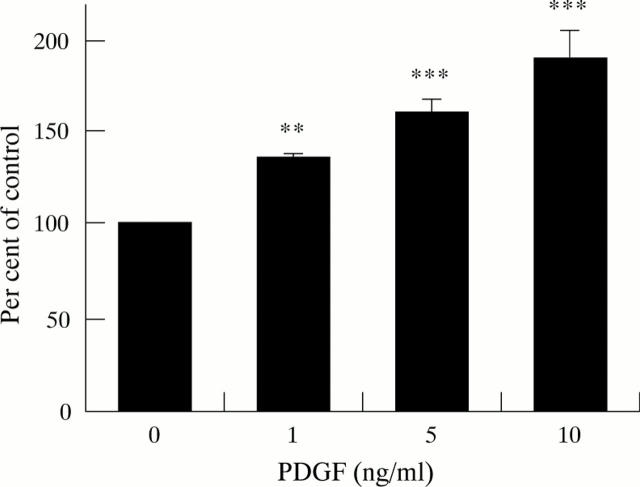
Figure 3 .
Effect of PDGF on DNA synthesis (five separate cell preparations). DNA synthesis was estimated by measuring the incorporation of 3H-thymidine into TCA precipitable material. Results are expressed as a percentage of control values observed in cells not incubated with PDGF. **p<0.01, ***p<0.001.
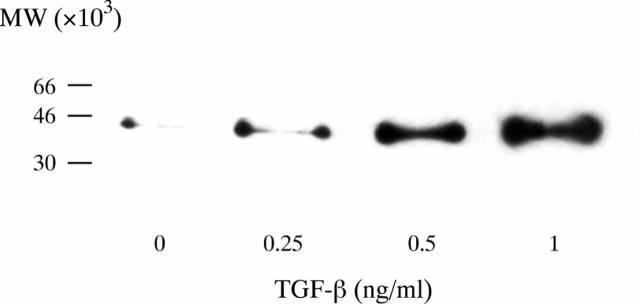
Figure 4 .
Western blot analysis of cell lysates for αSMA expression. The figure shows a representative immunoblot for expression in cells incubated with and without TGF-β1. A single band was detected in each lane corresponding to the known molecular weight (42 kDa) of αSMA.
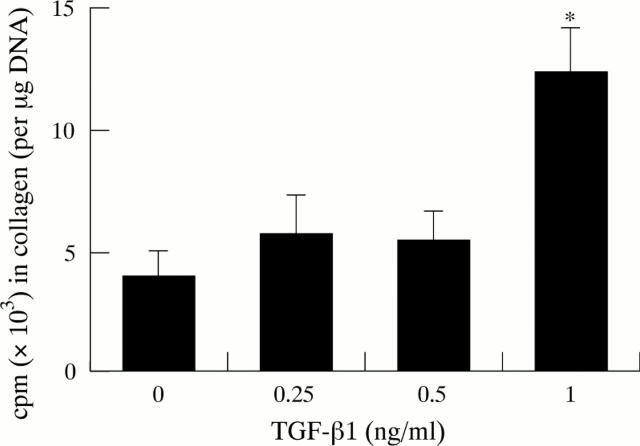
Figure 5 .
Effect of TGF-β1 on collagen synthesis (five separate cell preparations). *p<0.005.
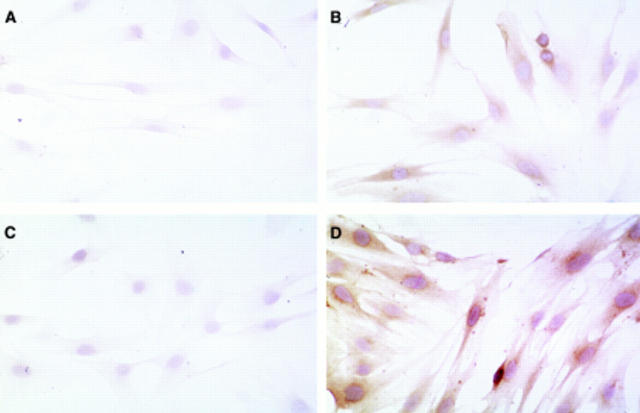
Figure 6 .
Effect of TGF-β1 on expression of the β subunit of the PDGF receptor (PDGF-Rβ). Panels A and B show stellate cells (not exposed to TGF-β1) incubated with non-immune serum (negative control) and an antibody to PDGF-Rβ respectively. Panels C and D show TGF-β1 treated stellate cells incubated with non-immune serum (negative control) and an antibody to PDGF-Rβ respectively.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apte M. V., Haber P. S., Applegate T. L., Norton I. D., McCaughan G. W., Korsten M. A., Pirola R. C., Wilson J. S. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998 Jul;43(1):128–133. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachem M. G., Meyer D., Melchior R., Sell K. M., Gressner A. M. Activation of rat liver perisinusoidal lipocytes by transforming growth factors derived from myofibroblastlike cells. A potential mechanism of self perpetuation in liver fibrogenesis. J Clin Invest. 1992 Jan;89(1):19–27. doi: 10.1172/JCI115561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedossa P., Paradis V. Transforming growth factor-beta (TGF-beta): a key-role in liver fibrogenesis. J Hepatol. 1995;22(2 Suppl):37–42. [PubMed] [Google Scholar]
- Braganza J. M., Scott P., Bilton D., Schofield D., Chaloner C., Shiel N., Hunt L. P., Bottiglieri T. Evidence for early oxidative stress in acute pancreatitis. Clues for correction. Int J Pancreatol. 1995 Feb;17(1):69–81. doi: 10.1007/BF02788361. [DOI] [PubMed] [Google Scholar]
- Burt A. D. C. L. Oakley Lecture (1993). Cellular and molecular aspects of hepatic fibrosis. J Pathol. 1993 Jun;170(2):105–114. doi: 10.1002/path.1711700203. [DOI] [PubMed] [Google Scholar]
- Day C. P. Is necroinflammation a prerequisite for fibrogenesis? Hepatogastroenterology. 1996 Jan-Feb;43(7):104–120. [PubMed] [Google Scholar]
- Farkas G. Gyulladáskeltö mediátorok heveny hasnyálmirigy-gyulladásban (elméleti megfontolások). Orv Hetil. 1995 Aug 20;136(34):1819–1822. [PubMed] [Google Scholar]
- Foidart J. M., Bere E. W., Jr, Yaar M., Rennard S. I., Gullino M., Martin G. R., Katz S. I. Distribution and immunoelectron microscopic localization of laminin, a noncollagenous basement membrane glycoprotein. Lab Invest. 1980 Mar;42(3):336–342. [PubMed] [Google Scholar]
- Friedman S. L., Arthur M. J. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest. 1989 Dec;84(6):1780–1785. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. L. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993 Jun 24;328(25):1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- Gress T., Müller-Pillasch F., Elsässer H. P., Bachem M., Ferrara C., Weidenbach H., Lerch M., Adler G. Enhancement of transforming growth factor beta 1 expression in the rat pancreas during regeneration from caerulein-induced pancreatitis. Eur J Clin Invest. 1994 Oct;24(10):679–685. doi: 10.1111/j.1365-2362.1994.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Gressner A. M., Bachem M. G. Molecular mechanisms of liver fibrogenesis--a homage to the role of activated fat-storing cells. Digestion. 1995;56(5):335–346. doi: 10.1159/000201257. [DOI] [PubMed] [Google Scholar]
- Gressner A. M. Cytokines and cellular crosstalk involved in the activation of fat-storing cells. J Hepatol. 1995;22(2 Suppl):28–36. [PubMed] [Google Scholar]
- Gressner A. M., Schäfer S. Comparison of sulphated glycosaminoglycan and hyaluronate synthesis and secretion in cultured hepatocytes, fat storing cells, and Kupffer cells. J Clin Chem Clin Biochem. 1989 Mar;27(3):141–149. doi: 10.1515/cclm.1989.27.3.141. [DOI] [PubMed] [Google Scholar]
- Jakowlew S. B., Mead J. E., Danielpour D., Wu J., Roberts A. B., Fausto N. Transforming growth factor-beta (TGF-beta) isoforms in rat liver regeneration: messenger RNA expression and activation of latent TGF-beta. Cell Regul. 1991 Jul;2(7):535–548. doi: 10.1091/mbc.2.7.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowitz P., Kratzer W., Zemmler T., Tudyka J., Wechsler J. G. Gallbladder sludge: spontaneous course and incidence of complications in patients without stones. Hepatology. 1994 Aug;20(2):291–294. [PubMed] [Google Scholar]
- Kapuściński J., Skoczylas B. Simple and rapid fluorimetric method for DNA microassay. Anal Biochem. 1977 Nov;83(1):252–257. doi: 10.1016/0003-2697(77)90533-4. [DOI] [PubMed] [Google Scholar]
- Kusske A. M., Rongione A. J., Reber H. A. Cytokines and acute pancreatitis. Gastroenterology. 1996 Feb;110(2):639–642. doi: 10.1053/gast.1996.v110.agast960639. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsuoka M., Pham N. T., Tsukamoto H. Differential effects of interleukin-1 alpha, tumor necrosis factor alpha, and transforming growth factor beta 1 on cell proliferation and collagen formation by cultured fat-storing cells. Liver. 1989 Apr;9(2):71–78. doi: 10.1111/j.1600-0676.1989.tb00382.x. [DOI] [PubMed] [Google Scholar]
- Menke A., Yamaguchi H., Gress T. M., Adler G. Extracellular matrix is reduced by inhibition of transforming growth factor beta1 in pancreatitis in the rat. Gastroenterology. 1997 Jul;113(1):295–303. doi: 10.1016/s0016-5085(97)70107-0. [DOI] [PubMed] [Google Scholar]
- Menè P., Abboud H. E., Dubyak G. R., Scarpa A., Dunn M. J. Effects of PDGF on inositol phosphates, Ca2+, and contraction of mesangial cells. Am J Physiol. 1987 Sep;253(3 Pt 2):F458–F463. doi: 10.1152/ajprenal.1987.253.3.F458. [DOI] [PubMed] [Google Scholar]
- Norton I. D., Apte M. V., Lux O., Haber P. S., Pirola R. C., Wilson J. S. Chronic ethanol administration causes oxidative stress in the rat pancreas. J Lab Clin Med. 1998 May;131(5):442–446. doi: 10.1016/s0022-2143(98)90145-7. [DOI] [PubMed] [Google Scholar]
- Parola M., Pinzani M., Casini A., Albano E., Poli G., Gentilini A., Gentilini P., Dianzani M. U. Stimulation of lipid peroxidation or 4-hydroxynonenal treatment increases procollagen alpha 1 (I) gene expression in human liver fat-storing cells. Biochem Biophys Res Commun. 1993 Aug 16;194(3):1044–1050. doi: 10.1006/bbrc.1993.1927. [DOI] [PubMed] [Google Scholar]
- Pinzani M., Gentilini A., Caligiuri A., De Franco R., Pellegrini G., Milani S., Marra F., Gentilini P. Transforming growth factor-beta 1 regulates platelet-derived growth factor receptor beta subunit in human liver fat-storing cells. Hepatology. 1995 Jan;21(1):232–239. [PubMed] [Google Scholar]
- Pinzani M., Gesualdo L., Sabbah G. M., Abboud H. E. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clin Invest. 1989 Dec;84(6):1786–1793. doi: 10.1172/JCI114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzani M., Knauss T. C., Pierce G. F., Hsieh P., Kenney W., Dubyak G. R., Abboud H. E. Mitogenic signals for platelet-derived growth factor isoforms in liver fat-storing cells. Am J Physiol. 1991 Mar;260(3 Pt 1):C485–C491. doi: 10.1152/ajpcell.1991.260.3.C485. [DOI] [PubMed] [Google Scholar]
- Pinzani M., Milani S., Grappone C., Weber F. L., Jr, Gentilini P., Abboud H. E. Expression of platelet-derived growth factor in a model of acute liver injury. Hepatology. 1994 Mar;19(3):701–707. doi: 10.1002/hep.1840190323. [DOI] [PubMed] [Google Scholar]
- Pinzani M., Milani S., Herbst H., DeFranco R., Grappone C., Gentilini A., Caligiuri A., Pellegrini G., Ngo D. V., Romanelli R. G. Expression of platelet-derived growth factor and its receptors in normal human liver and during active hepatic fibrogenesis. Am J Pathol. 1996 Mar;148(3):785–800. [PMC free article] [PubMed] [Google Scholar]
- Ross R. Platelet-derived growth factor. Lancet. 1989 May 27;1(8648):1179–1182. doi: 10.1016/s0140-6736(89)92760-8. [DOI] [PubMed] [Google Scholar]
- Schäfer S., Zerbe O., Gressner A. M. The synthesis of proteoglycans in fat-storing cells of rat liver. Hepatology. 1987 Jul-Aug;7(4):680–687. doi: 10.1002/hep.1840070411. [DOI] [PubMed] [Google Scholar]
- Van Laethem J. L., Robberecht P., Résibois A., Devière J. Transforming growth factor beta promotes development of fibrosis after repeated courses of acute pancreatitis in mice. Gastroenterology. 1996 Feb;110(2):576–582. doi: 10.1053/gast.1996.v110.pm8566606. [DOI] [PubMed] [Google Scholar]
- Wahl M. I., Olashaw N. E., Nishibe S., Rhee S. G., Pledger W. J., Carpenter G. Platelet-derived growth factor induces rapid and sustained tyrosine phosphorylation of phospholipase C-gamma in quiescent BALB/c 3T3 cells. Mol Cell Biol. 1989 Jul;9(7):2934–2943. doi: 10.1128/mcb.9.7.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Laethem J. L., Deviere J., Resibois A., Rickaert F., Vertongen P., Ohtani H., Cremer M., Miyazono K., Robberecht P. Localization of transforming growth factor beta 1 and its latent binding protein in human chronic pancreatitis. Gastroenterology. 1995 Jun;108(6):1873–1881. doi: 10.1016/0016-5085(95)90152-3. [DOI] [PubMed] [Google Scholar]