Abstract
AIMS—To study changes in the expression of insulin-like growth factors (IGFs) and their receptors, as well as production of the IGF-I and IGF-II polypeptides, in adenocarcinoma of the colon. METHODS—Malignant tissue obtained at operation was used. Total RNA was extracted and specific IGF-I and IGF-II and their receptor mRNAs were measured by a solution hybridisation RNase protection assay. IGF-I and IGF-II polypeptides were measured by specific immunoassays. RESULTS—All normal tissues expressed IGF-II, IGF-I receptor, and IGF-II/mannose-6-phosphate (Man-6-P) receptor. IGF-I mRNA could not be detected but the polypeptide was present in small but equal amounts in normal and malignant tissue. IGF-II was expressed 40 times more abundantly in colonic tumours than in adjacent normal tissue and the concentration of the corresponding polypeptide was twice as high in the malignant tissue. IGF-I receptor expression was increased by a factor of 2.5 and IGF-II/Man-6-P receptor by a factor of 4. CONCLUSIONS—This study confirms that in adenocarcinoma of the human colon there is increased expression of IGF-I receptor and IGF-II. Furthermore, IGF-II/Man-6-P receptor message is increased and the increase in IGF-II message is accompanied by a doubling of the IGF-II protein in the tumour tissue compared with the adjacent normal tissue. These findings suggest that the IGF-II/Man-6-P receptor may also be involved in development of adenocarcinoma of the colon. There is rapidly accumulating evidence implicating the IGF system in the development of malignancy of the large bowel.
Keywords: insulin-like growth factors; adenocarcinoma; colon
Full Text
The Full Text of this article is available as a PDF (113.6 KB).
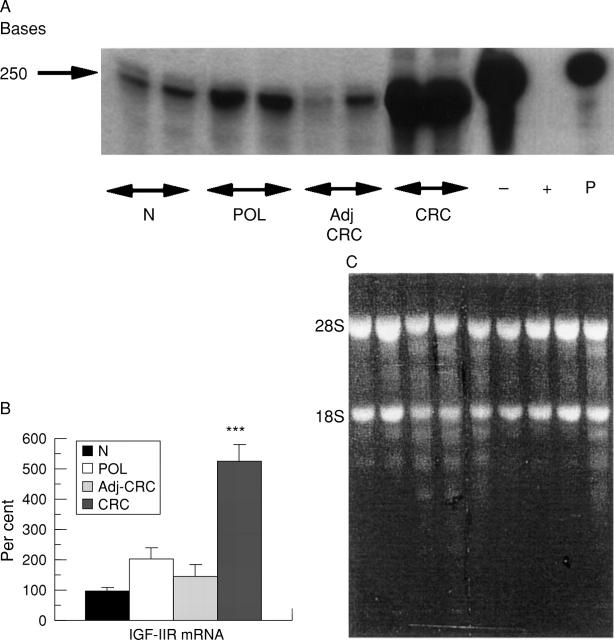
Figure 1 .
Expression of insulin-like growth factor II (IGF-II)/mannose-6-phosphate (Man-6-P) receptor in colonic tissue. (A) Levels of IGF-II/Man-6-P receptor mRNA in various groups of individuals are compared. The first two lanes are from two healthy individuals (N) followed by two lanes from one patient with a malignant polyp (POL). The next four lanes are from one patient with colonic cancer. The first two are from normal tissue adjacent to the carcinoma (Adj-CRC) and the last two from the tumour itself (CRC). − denotes yeast RNA and probe without RNase, and + denotes yeast RNA and probe with RNase; P denotes native probe. The levels of the IGF-II/Man-6-P receptor mRNA were measured by solution hybridisation RNase protection assay as described in Methods. The gels were exposed for five days. (B) Expression of IGF-II/Man-6-P receptor (IGF-IIR) mRNA in 10 specimens obtained from 10 healthy individuals (N), in four specimens from three patients with a malignant polyp (POL), in nine specimens from six individuals of tissue adjacent to adenocarcinoma (Adj-CRC), and malignant tissue from the same six individuals (CRC). There was a highly significant difference between adenocarcinoma of the colon and the other groups (***p<0.001). mRNA levels were measured by solution hybridisation RNase protection assay as described in Methods. The values are the relative density of the bands as a percentage of the normal tissue. Results are mean (SEM). (C) The integrity of the RNA from these individuals was assessed by visual inspection of ethidium bromide stained 28S and 18S ribosomal bands after agarose gel electrophoresis of 10 µg aliquots.
Figure 2 .
Expression of insulin-like growth factor-II (IGF-II) mRNA in adenocarcinoma of the colon (CRC) compared with that in tissue adjacent to the carcinoma (Adj-CRC). mRNA levels were measured by solution hybridisation RNase protection assay as described in Methods. The values are the relative density of the bands as percentage of the normal adjacent tissue coming from six individuals and specimens of adenocarcinoma obtained from the same individuals. There was a highly significant difference (***p<0.001) between the two groups. Results are mean (SEM).
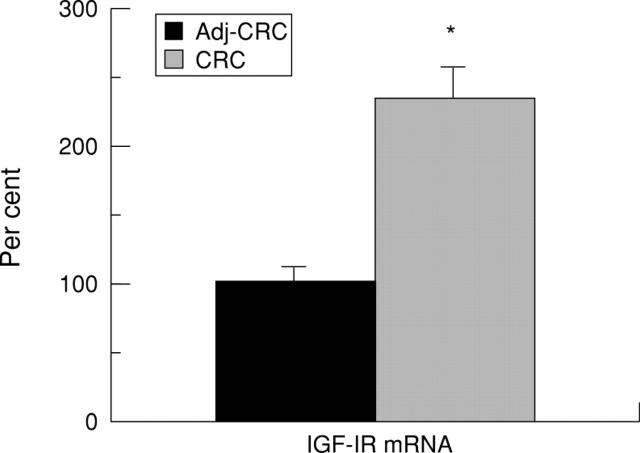
Figure 3 .
Expression of insulin-like growth factor-I receptor (IGF-IR) mRNA in adenocarcinoma of the colon (CRC) compared with that in tissue adjacent to the carcinoma (Adj-CRC). mRNA levels were measured by solution hybridisation RNase protection assay as described in Methods. The values are the relative density of the bands as percentage of the normal adjacent tissue consisting of 12 specimens from six individuals and 12 specimens of tissue from the adenocarcinoma obtained from the same six individuals. Results are mean (SEM). There was a highly significant difference (*p<0.0001) between the two groups.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Ahuja N., Mohan A. L., Li Q., Stolker J. M., Herman J. G., Hamilton S. R., Baylin S. B., Issa J. P. Association between CpG island methylation and microsatellite instability in colorectal cancer. Cancer Res. 1997 Aug 15;57(16):3370–3374. [PubMed] [Google Scholar]
- Bates P., Fisher R., Ward A., Richardson L., Hill D. J., Graham C. F. Mammary cancer in transgenic mice expressing insulin-like growth factor II (IGF-II) Br J Cancer. 1995 Nov;72(5):1189–1193. doi: 10.1038/bjc.1995.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- D'Ercole A. J., Stiles A. D., Underwood L. E. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci U S A. 1984 Feb;81(3):935–939. doi: 10.1073/pnas.81.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza A. T., Hankins G. R., Washington M. K., Orton T. C., Jirtle R. L. M6P/IGF2R gene is mutated in human hepatocellular carcinomas with loss of heterozygosity. Nat Genet. 1995 Dec;11(4):447–449. doi: 10.1038/ng1295-447. [DOI] [PubMed] [Google Scholar]
- Flyvbjerg A., Thorlacius-Ussing O., Naeraa R., Ingerslev J., Orskov H. Kidney tissue somatomedin C and initial renal growth in diabetic and uninephrectomized rats. Diabetologia. 1988 May;31(5):310–314. doi: 10.1007/BF00277413. [DOI] [PubMed] [Google Scholar]
- Garrouste F., Remacle-Bonnet M., Culouscou J. M., Marvaldi J., Pommier G. Type-II insulin-like growth-factor receptor in conditioned medium from HT-29 human colon carcinoma cell line. Int J Cancer. 1991 Mar 12;47(5):760–764. doi: 10.1002/ijc.2910470523. [DOI] [PubMed] [Google Scholar]
- Guo Y. S., Jin G. F., Townsend C. M., Jr, Zhang T., Sheng H. M., Beauchamp R. D., Thompson J. C. Insulin-like growth factor-II expression in carcinoma in colon cell lines: implications for autocrine actions. J Am Coll Surg. 1995 Aug;181(2):145–154. [PubMed] [Google Scholar]
- Guo Y. S., Narayan S., Yallampalli C., Singh P. Characterization of insulinlike growth factor I receptors in human colon cancer. Gastroenterology. 1992 Apr;102(4 Pt 1):1101–1108. [PubMed] [Google Scholar]
- Hankins G. R., De Souza A. T., Bentley R. C., Patel M. R., Marks J. R., Iglehart J. D., Jirtle R. L. M6P/IGF2 receptor: a candidate breast tumor suppressor gene. Oncogene. 1996 May 2;12(9):2003–2009. [PubMed] [Google Scholar]
- Heinz-Erian P., Kessler U., Funk B., Gais P., Kiess W. Identification and in situ localization of the insulin-like growth factor-II/mannose-6-phosphate (IGF-II/M6P) receptor in the rat gastrointestinal tract: comparison with the IGF-I receptor. Endocrinology. 1991 Oct;129(4):1769–1778. doi: 10.1210/endo-129-4-1769. [DOI] [PubMed] [Google Scholar]
- Hernandez E. R., Hurwitz A., Vera A., Pellicer A., Adashi E. Y., LeRoith D., Roberts C. T., Jr Expression of the genes encoding the insulin-like growth factors and their receptors in the human ovary. J Clin Endocrinol Metab. 1992 Feb;74(2):419–425. doi: 10.1210/jcem.74.2.1309838. [DOI] [PubMed] [Google Scholar]
- Ionov Y., Peinado M. A., Malkhosyan S., Shibata D., Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993 Jun 10;363(6429):558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- Lambert S., Vivario J., Boniver J., Gol-Winkler R. Abnormal expression and structural modification of the insulin-like growth-factor-II gene in human colorectal tumors. Int J Cancer. 1990 Sep 15;46(3):405–410. doi: 10.1002/ijc.2910460313. [DOI] [PubMed] [Google Scholar]
- Lamonerie T., Lavialle C., Haddada H., Brison O. IGF-2 autocrine stimulation in tumorigenic clones of a human colon-carcinoma cell line. Int J Cancer. 1995 May 16;61(4):587–592. doi: 10.1002/ijc.2910610425. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bowsher R. R., Apathy J. M., Smith M. C., Henry D. P. Measurement of insulin-like growth factor-II in physiological fluids and tissues. II. Extraction quantification in rat tissues. Endocrinology. 1991 Feb;128(2):815–822. doi: 10.1210/endo-128-2-815. [DOI] [PubMed] [Google Scholar]
- Lowe W. L., Jr, Roberts C. T., Jr, LeRoith D., Rojeski M. T., Merimee T. J., Fui S. T., Keen H., Arnold D., Mersey J., Gluzman S. Insulin-like growth factor-II in nonislet cell tumors associated with hypoglycemia: increased levels of messenger ribonucleic acid. J Clin Endocrinol Metab. 1989 Dec;69(6):1153–1159. doi: 10.1210/jcem-69-6-1153. [DOI] [PubMed] [Google Scholar]
- MacDonald R. S., Park J. H., Thornton W. H., Jr Insulin, IGF-1, and IGF-2 receptors in rat small intestine following massive small bowel resection. Analysis by binding, flow cytometry, and immunohistochemistry. Dig Dis Sci. 1993 Sep;38(9):1658–1669. doi: 10.1007/BF01303175. [DOI] [PubMed] [Google Scholar]
- Morgan D. O., Edman J. C., Standring D. N., Fried V. A., Smith M. C., Roth R. A., Rutter W. J. Insulin-like growth factor II receptor as a multifunctional binding protein. Nature. 1987 Sep 24;329(6137):301–307. doi: 10.1038/329301a0. [DOI] [PubMed] [Google Scholar]
- Mulroney S. E., Haramati A., Werner H., Bondy C., Roberts C. T., Jr, LeRoith D. Altered expression of insulin-like growth factor-I (IGF-I) and IGF receptor genes after unilateral nephrectomy in immature rats. Endocrinology. 1992 Jan;130(1):249–256. doi: 10.1210/endo.130.1.1309331. [DOI] [PubMed] [Google Scholar]
- Singh P., Dai B., Yallampalli C., Xu Z. Expression of IGF-II and IGF-binding proteins by colon cancer cells in relation to growth response to IGFs. Am J Physiol. 1994 Oct;267(4 Pt 1):G608–G617. doi: 10.1152/ajpgi.1994.267.4.G608. [DOI] [PubMed] [Google Scholar]
- Singh P., Dai B., Yallampalli U., Lu X., Schroy P. C. Proliferation and differentiation of a human colon cancer cell line (CaCo2) is associated with significant changes in the expression and secretion of insulin-like growth factor (IGF) IGF-II and IGF binding protein-4: role of IGF-II. Endocrinology. 1996 May;137(5):1764–1774. doi: 10.1210/endo.137.5.8612513. [DOI] [PubMed] [Google Scholar]
- Singh P., Rubin N. Insulinlike growth factors and binding proteins in colon cancer. Gastroenterology. 1993 Oct;105(4):1218–1237. doi: 10.1016/0016-5085(93)90971-e. [DOI] [PubMed] [Google Scholar]
- Thissen J. P., Ketelslegers J. M., Underwood L. E. Nutritional regulation of the insulin-like growth factors. Endocr Rev. 1994 Feb;15(1):80–101. doi: 10.1210/edrv-15-1-80. [DOI] [PubMed] [Google Scholar]
- Tricoli J. V., Rall L. B., Karakousis C. P., Herrera L., Petrelli N. J., Bell G. I., Shows T. B. Enhanced levels of insulin-like growth factor messenger RNA in human colon carcinomas and liposarcomas. Cancer Res. 1986 Dec;46(12 Pt 1):6169–6173. [PubMed] [Google Scholar]
- Ulshen M. H., Dowling R. H., Fuller C. R., Zimmermann E. M., Lund P. K. Enhanced growth of small bowel in transgenic mice overexpressing bovine growth hormone. Gastroenterology. 1993 Apr;104(4):973–980. doi: 10.1016/0016-5085(93)90263-c. [DOI] [PubMed] [Google Scholar]
- Vanderhoof J. A. Regulatory peptides and intestinal growth. Gastroenterology. 1993 Apr;104(4):1205–1208. doi: 10.1016/0016-5085(93)90295-n. [DOI] [PubMed] [Google Scholar]
- Yee D., Morales F. R., Hamilton T. C., Von Hoff D. D. Expression of insulin-like growth factor I, its binding proteins, and its receptor in ovarian cancer. Cancer Res. 1991 Oct 1;51(19):5107–5112. [PubMed] [Google Scholar]
- Zarrilli R., Pignata S., Romano M., Gravina A., Casola S., Bruni C. B., Acquaviva A. M. Expression of insulin-like growth factor (IGF)-II and IGF-I receptor during proliferation and differentiation of CaCo-2 human colon carcinoma cells. Cell Growth Differ. 1994 Oct;5(10):1085–1091. [PubMed] [Google Scholar]