Abstract
BACKGROUND—Many β lactams are well absorbed by the small intestine, although the reasons for this are poorly understood. AIMS—To characterise the uptake of penicillin G into human small intestinal brush border membrane vesicles (BBMV) and to compare the uptake characteristics to those of rabbit BBMV. METHODS AND RESULTS—Uptake of penicillin G was studied in human BBMV. Penicillin G was actively transported into the lumen of BBMV via an H+ dependent, Na+ independent uptake system. The carrier mediated process was saturable and adhered to Michaelis-Menten kinetics (Vmax 52 nmol penicillin G per mg protein per 30 seconds, Km 13.9 mM). These results are similar to those previously reported in rabbit BBMV. CONCLUSIONS—It is suggested that penicillin G can be used as a model molecule for peptide and β lactam transport studies as it is cheap and readily available in isotopically labelled form. Furthermore, rabbit BBMV may be used as an acceptable substitute for human BBMV for the study of penicillin transport.
Keywords: brush border membrane vesicles; β lactam; antibiotic uptake; oral administration; pH gradient
Full Text
The Full Text of this article is available as a PDF (146.0 KB).
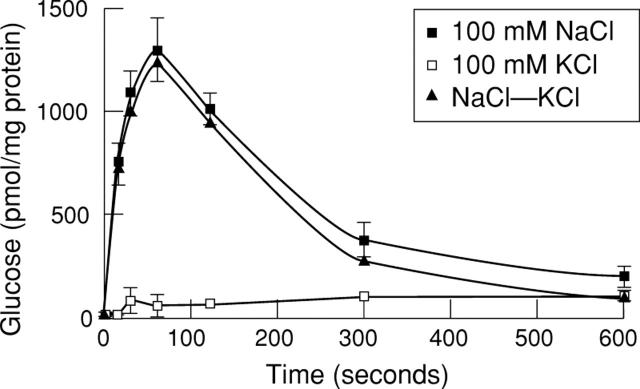
Figure 1 .
Time course of glucose uptake in hBBMV. Vesicles were loaded with 5 mM HEPES/Tris, 0.1 mM MgSO4, 100 mM mannitol, 100 mM KCl at pH 7.1. The assay mixture contained 100 mM NaCl or 100 mM KCl, 100 mM mannitol, 0.1 mM glucose, and 5 mM HEPES/Tris pH 7.1. Samples were incubated at 37°C. Results represent the average of three experiments carried out in triplicate. Error bars represent standard deviation.
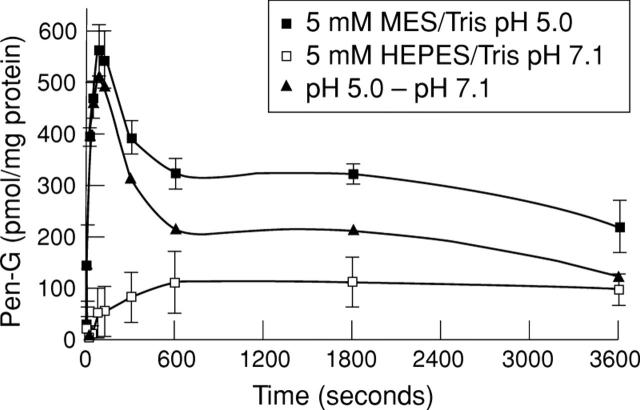
Figure 2 .
Time course of penicillin G uptake in hBBMV. Vesicles were loaded with 5 mM HEPES/Tris, 0.1 mM MgSO4, 100 mM mannitol, 100 mM KCl at pH 7.1. The assay mixture contained 100 mM KCl, 100 mM mannitol, 0.1 mM penicillin G, and 5 mM MES/Tris pH 5.0, or 5 mM HEPES/Tris for pH 7.1, respectively. Samples were incubated at 37°C. Results represent the average of three distinct experiments carried out in triplicate. Error bars represent standard deviation.
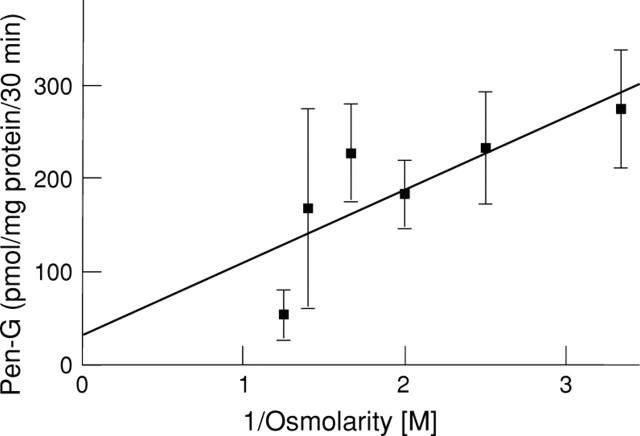
Figure 3 .
Effect of osmolarity on penicillin G uptake in hBBMV. Vesicles were loaded with 50 mM HEPES/Tris, 0.1 mM MgSO4, 50 mM mannitol, 100 mM KCl at pH 7.1. The external buffer contained 100 mM KCl, 50-550 mM mannitol, 50 mM MES/Tris pH 5.0 and 0.1 mM penicillin G. Samples were incubated for 30 minutes at 37°C. The results represent the average of two distinct experiments carried out in triplicate. Error bars represent standard deviation. Intercept on y axis represents the amount of penicillin G associated with the membrane (12%); r=0.791.
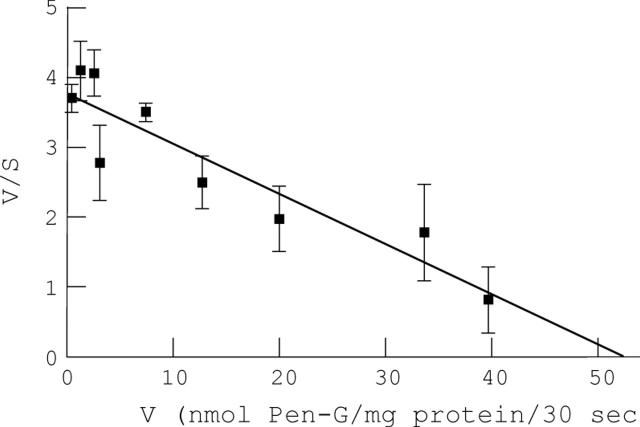
Figure 4 .
Eadie-Hofstee plot of penicillin G uptake in hBBMV. Vesicles were loaded with 50 mM HEPES/Tris, 0.1 mM MgSO4, 50 mM mannitol, 100 mM KCl at pH 7.1. The external buffer contained 100 mM KCl, 0-50 mM mannitol (to correct for penicillin G addition), 50 mM MES/Tris pH 5.0, and 0.1-50 mM penicillin G. Samples were incubated for 30 seconds at 37°C. Results represent the average of three distinct experiments carried out in triplicate. Error bars represent standard deviation. Vmax = 52 nmol/mg protein/30 sec; Km = 13.9 mM; r=0.924.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison J. M., Burston D., Dalrymple J. A., Matthews D. M., Payne J. W., Sleisenger M. H., Wilkinson S. A common mechanism for transport of di- and tri-peptides by hamster jejunum in vitro. Clin Sci Mol Med. 1975 Oct;49(4):313–322. doi: 10.1042/cs0490313. [DOI] [PubMed] [Google Scholar]
- Addison J. M., Burston D., Matthews D. M. Evidence for active transport of the dipeptide glycylsarcosine by hamster jejunum in vitro. Clin Sci. 1972 Dec;43(6):907–911. doi: 10.1042/cs0430907. [DOI] [PubMed] [Google Scholar]
- Addison J. M., Burston D., Payne J. W., Wilkinson S., Matthews D. M. Evidence for active transport of tripeptides by hamster jejunum in vitro. Clin Sci Mol Med. 1975 Oct;49(4):305–312. doi: 10.1042/cs0490305. [DOI] [PubMed] [Google Scholar]
- Bergan T. Pharmacokinetic properties of the cephalosporins. Drugs. 1987;34 (Suppl 2):89–104. doi: 10.2165/00003495-198700342-00008. [DOI] [PubMed] [Google Scholar]
- Brandsch M., Brandsch C., Ganapathy M. E., Chew C. S., Ganapathy V., Leibach F. H. Influence of proton and essential histidyl residues on the transport kinetics of the H+/peptide cotransport systems in intestine (PEPT 1) and kidney (PEPT 2). Biochim Biophys Acta. 1997 Mar 13;1324(2):251–262. doi: 10.1016/s0005-2736(96)00231-3. [DOI] [PubMed] [Google Scholar]
- Colas B., Maroux S. Simultaneous isolation of brush border and basolateral membrane from rabbit enterocytes. Presence of brush border hydrolases in the basolateral membrane of rabbit enterocytes. Biochim Biophys Acta. 1980 Aug 4;600(2):406–420. doi: 10.1016/0005-2736(80)90444-7. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- Dantzig A. H., Hoskins J. A., Tabas L. B., Bright S., Shepard R. L., Jenkins I. L., Duckworth D. C., Sportsman J. R., Mackensen D., Rosteck P. R., Jr Association of intestinal peptide transport with a protein related to the cadherin superfamily. Science. 1994 Apr 15;264(5157):430–433. doi: 10.1126/science.8153632. [DOI] [PubMed] [Google Scholar]
- Fairclough P., Malathi P., Preiser H., Crane R. K. Reconstitution into liposomes of glucose active transport from the rabbit renal proximal tubule. Characteristics of the system. Biochim Biophys Acta. 1979 May 17;553(2):295–306. doi: 10.1016/0005-2736(79)90233-5. [DOI] [PubMed] [Google Scholar]
- Fei Y. J., Kanai Y., Nussberger S., Ganapathy V., Leibach F. H., Romero M. F., Singh S. K., Boron W. F., Hediger M. A. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature. 1994 Apr 7;368(6471):563–566. doi: 10.1038/368563a0. [DOI] [PubMed] [Google Scholar]
- Feracci H., Maroux S. Rabbit intestinal aminopeptidase N. Purification and molecular properties. Biochim Biophys Acta. 1980 Jul;599(2):448–463. doi: 10.1016/0005-2736(80)90190-x. [DOI] [PubMed] [Google Scholar]
- Ganapathy, Leibach F. H. Is intestinal peptide transport energized by a proton gradient? Am J Physiol. 1985 Aug;249(2 Pt 1):G153–G160. doi: 10.1152/ajpgi.1985.249.2.G153. [DOI] [PubMed] [Google Scholar]
- Ganapathy M. E., Prasad P. D., Mackenzie B., Ganapathy V., Leibach F. H. Interaction of anionic cephalosporins with the intestinal and renal peptide transporters PEPT 1 and PEPT 2. Biochim Biophys Acta. 1997 Mar 13;1324(2):296–308. doi: 10.1016/s0005-2736(96)00234-9. [DOI] [PubMed] [Google Scholar]
- Ganapathy V., Mendicino J. F., Leibach F. H. Transport of glycyl-L-proline into intestinal and renal brush border vesicles from rabbit. J Biol Chem. 1981 Jan 10;256(1):118–124. [PubMed] [Google Scholar]
- Himukai M., Hoshi T. Mechanisms of glycyl-L-leucine uptake by guinea-pig small intestine: relative importance of intact-peptide transport. J Physiol. 1980 May;302:155–169. doi: 10.1113/jphysiol.1980.sp013235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui K., Okano T., Maegawa H., Kato M., Takano M., Hori R. H+ coupled transport of p.o. cephalosporins via dipeptide carriers in rabbit intestinal brush-border membranes: difference of transport characteristics between cefixime and cephradine. J Pharmacol Exp Ther. 1988 Oct;247(1):235–241. [PubMed] [Google Scholar]
- Iseki K., Sugawara M., Saitoh H., Miyazaki K., Arita T. Comparison of transport characteristics of amino beta-lactam antibiotics and dipeptides across rat intestinal brush border membrane. J Pharm Pharmacol. 1989 Sep;41(9):628–632. doi: 10.1111/j.2042-7158.1989.tb06544.x. [DOI] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Kimura T., Yamamoto T., Ishizuka R., Sezaki H. Transport of cefadroxil, an aminocephalosporin antibiotic, across the small intestinal brush border membrane. Biochem Pharmacol. 1985 Jan 1;34(1):81–84. doi: 10.1016/0006-2952(85)90103-0. [DOI] [PubMed] [Google Scholar]
- Kramer W., Dechent C., Girbig F., Gutjahr U., Neubauer H. Intestinal uptake of dipeptides and beta-lactam antibiotics. I. The intestinal uptake system for dipeptides and beta-lactam antibiotics is not part of a brush border membrane peptidase. Biochim Biophys Acta. 1990 Nov 30;1030(1):41–49. doi: 10.1016/0005-2736(90)90236-h. [DOI] [PubMed] [Google Scholar]
- Kramer W., Girbig F., Leipe I., Petzoldt E. Direct photoaffinity labelling of binding proteins for beta-lactam antibiotics in rabbit intestinal brush border membranes with [3H]benzylpenicillin. Biochem Pharmacol. 1988 Jun 15;37(12):2427–2435. doi: 10.1016/0006-2952(88)90370-x. [DOI] [PubMed] [Google Scholar]
- Liang R., Fei Y. J., Prasad P. D., Ramamoorthy S., Han H., Yang-Feng T. L., Hediger M. A., Ganapathy V., Leibach F. H. Human intestinal H+/peptide cotransporter. Cloning, functional expression, and chromosomal localization. J Biol Chem. 1995 Mar 24;270(12):6456–6463. doi: 10.1074/jbc.270.12.6456. [DOI] [PubMed] [Google Scholar]
- Liu W., Liang R., Ramamoorthy S., Fei Y. J., Ganapathy M. E., Hediger M. A., Ganapathy V., Leibach F. H. Molecular cloning of PEPT 2, a new member of the H+/peptide cotransporter family, from human kidney. Biochim Biophys Acta. 1995 May 4;1235(2):461–466. doi: 10.1016/0005-2736(95)80036-f. [DOI] [PubMed] [Google Scholar]
- Lowther J., Hammond S. M., Russell K., Fairclough P. D. Uptake of cephalosporins by human intestinal brush-border membrane vesicles. J Antimicrob Chemother. 1990 Jan;25(1):183–184. doi: 10.1093/jac/25.1.183. [DOI] [PubMed] [Google Scholar]
- Lucas M. L., Blair J. A., Cooper B., Matty A. J. Further investigations with pH microelectrodes into the jejunal microclimate in rat and man. Gut. 1975 Oct;16(10):844–844. [PubMed] [Google Scholar]
- Lucas M. Determination of acid surface pH in vivo in rat proximal jejunum. Gut. 1983 Aug;24(8):734–739. doi: 10.1136/gut.24.8.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D. M., Addison J. M., Burston D. Evidence for active transport of the dipeptide carnosine (beta-alanyl-L-histidine) by hamster jejunum in vitro. Clin Sci Mol Med. 1974 Jun;46(6):693–705. doi: 10.1042/cs0460693. [DOI] [PubMed] [Google Scholar]
- Minami H., Morse E. L., Adibi S. A. Characteristics and mechanism of glutamine-dipeptide absorption in human intestine. Gastroenterology. 1992 Jul;103(1):3–11. doi: 10.1016/0016-5085(92)91088-l. [DOI] [PubMed] [Google Scholar]
- Muranushi N., Yoshikawa T., Yoshida M., Oguma T., Hirano K., Yamada H. Transport characteristics of ceftibuten, a new oral cephem, in rat intestinal brush-border membrane vesicles: relationship to oligopeptide and amino beta-lactam transport. Pharm Res. 1989 Apr;6(4):308–312. doi: 10.1023/a:1015946407709. [DOI] [PubMed] [Google Scholar]
- Okano T., Inui K., Maegawa H., Takano M., Hori R. H+ coupled uphill transport of aminocephalosporins via the dipeptide transport system in rabbit intestinal brush-border membranes. J Biol Chem. 1986 Oct 25;261(30):14130–14134. [PubMed] [Google Scholar]
- Poschet J. F., Hammond S. M., Fairclough P. D. Characterisation of penicillin-G uptake in rabbit small-intestinal brush-border membrane vesicles. Biochim Biophys Acta. 1996 Jan 31;1278(2):233–240. doi: 10.1016/0005-2736(95)00226-x. [DOI] [PubMed] [Google Scholar]
- Rajendran V. M., Ansari S. A., Harig J. M., Adams M. B., Khan A. H., Ramaswamy K. Transport of glycyl-L-proline by human intestinal brush border membrane vesicles. Gastroenterology. 1985 Dec;89(6):1298–1304. doi: 10.1016/0016-5085(85)90646-8. [DOI] [PubMed] [Google Scholar]
- Rubino A., Field M., Shwachman H. Intestinal transport of amino acid residues of dipeptides. I. Influx of the glycine residue of glycyl-L-proline across mucosal border. J Biol Chem. 1971 Jun 10;246(11):3542–3548. [PubMed] [Google Scholar]
- Sabolić I., Burckhardt G. Effect of the preparation method on Na+-H+ exchange and ion permeabilities in rat renal brush-border membranes. Biochim Biophys Acta. 1984 May 16;772(2):140–148. doi: 10.1016/0005-2736(84)90037-3. [DOI] [PubMed] [Google Scholar]
- Shirazi-Beechey S. P., Davies A. G., Tebbutt K., Dyer J., Ellis A., Taylor C. J., Fairclough P., Beechey R. B. Preparation and properties of brush-border membrane vesicles from human small intestine. Gastroenterology. 1990 Mar;98(3):676–685. doi: 10.1016/0016-5085(90)90288-c. [DOI] [PubMed] [Google Scholar]
- Shirazi S. P., Beechey R. B., Butterworth P. J. The use of potent inhibitors of alkaline phosphatase to investigate the role of the enzyme in intestinal transport of inorganic phosphate. Biochem J. 1981 Mar 15;194(3):803–809. doi: 10.1042/bj1940803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sugawara M., Iseki K., Miyazaki K. H+ coupled transport of orally active cephalosporins lacking an alpha-amino group across brush-border membrane vesicles from rat small intestine. J Pharm Pharmacol. 1991 Jun;43(6):433–435. doi: 10.1111/j.2042-7158.1991.tb03504.x. [DOI] [PubMed] [Google Scholar]
- Sugawara M., Iseki K., Miyazaki K., Shiroto H., Kondo Y., Uchino J. Transport characteristics of ceftibuten, cefixime and cephalexin across human jejunal brush-border membrane. J Pharm Pharmacol. 1991 Dec;43(12):882–884. doi: 10.1111/j.2042-7158.1991.tb03203.x. [DOI] [PubMed] [Google Scholar]
- Sugawara M., Toda T., Iseki K., Miyazaki K., Shiroto H., Kondo Y., Uchino J. Transport characteristics of cephalosporin antibiotics across intestinal brush-border membrane in man, rat and rabbit. J Pharm Pharmacol. 1992 Dec;44(12):968–972. doi: 10.1111/j.2042-7158.1992.tb07075.x. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Hirooka H., Tamai I., Terasaki T. Evidence for a carrier-mediated transport system in the small intestine available for FK089, a new cephalosporin antibiotic without an amino group. J Antibiot (Tokyo) 1986 Nov;39(11):1592–1597. doi: 10.7164/antibiotics.39.1592. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Hirooka H., Terasaki T., Tamai I., Nakashima E. Saturable uptake of cefixime, a new oral cephalosporin without an alpha-amino group, by the rat intestine. J Pharm Pharmacol. 1987 Apr;39(4):272–277. doi: 10.1111/j.2042-7158.1987.tb06265.x. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Miyamoto E., Kubo O., Yamana T. GI absorption of beta-lactam antibiotics. III: Kinetic evidence for in situ absorption of ionized species of monobasic penicillins and cefazolin from the rat small intestine and structure-absorption rate relationships. J Pharm Sci. 1979 Jul;68(7):812–816. doi: 10.1002/jps.2600680706. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Tamai I., Hirooka H., Terasaki T. Beta-lactam antibiotics and transport via the dipeptide carrier system across the intestinal brush-border membrane. Biochem Pharmacol. 1987 Feb 15;36(4):565–567. doi: 10.1016/0006-2952(87)90369-8. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Terasaki T., Tamai I., Hirooka H. H+ gradient-dependent and carrier-mediated transport of cefixime, a new cephalosporin antibiotic, across brush-border membrane vesicles from rat small intestine. J Pharmacol Exp Ther. 1987 May;241(2):594–601. [PubMed] [Google Scholar]
- Westphal J. F., Deslandes A., Brogard J. M., Carbon C. Reappraisal of amoxycillin absorption kinetics. J Antimicrob Chemother. 1991 May;27(5):647–654. doi: 10.1093/jac/27.5.647. [DOI] [PubMed] [Google Scholar]
- Wilson D., Barry J. A., Ramaswamy K. Characteristics of tripeptide transport in human jejunal brush-border membrane vesicles. Biochim Biophys Acta. 1989 Nov 17;986(1):123–129. doi: 10.1016/0005-2736(89)90280-0. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T., Muranushi N., Yoshida M., Oguma T., Hirano K., Yamada H. Transport characteristics of ceftibuten (7432-S), a new oral cephem, in rat intestinal brush-border membrane vesicles: proton-coupled and stereoselective transport of ceftibuten. Pharm Res. 1989 Apr;6(4):302–307. doi: 10.1023/a:1015994323639. [DOI] [PubMed] [Google Scholar]
- Yuasa H., Amidon G. L., Fleisher D. Peptide carrier-mediated transport in intestinal brush border membrane vesicles of rats and rabbits: cephradine uptake and inhibition. Pharm Res. 1993 Mar;10(3):400–404. doi: 10.1023/a:1018940306394. [DOI] [PubMed] [Google Scholar]