Abstract
BACKGROUND—Non-steroidal anti-inflammatory drugs (NSAIDs) are effective for arthritis but cause gastrointestinal injury. Bovine colostrum is a rich source of growth factors and is marketed as a health food supplement. AIMS—To examine whether spray dried, defatted colostrum or milk preparations could reduce gastrointestinal injury caused by indomethacin. METHODS—Effects of test solutions, administered orally, were examined using an indomethacin restraint rat model of gastric damage and an indomethacin mouse model of small intestinal injury. Effects on migration of the human colonic carcinoma cell line HT-29 and rat small intestinal cell line RIE-1 were assessed using a wounded monolayer assay system (used as an in vitro model of wound repair) and effects on proliferation determined using [3H]thymidine incorporation. RESULTS—Pretreatment with 0.5 or 1 ml colostral preparation reduced gastric injury by 30% and 60% respectively in rats. A milk preparation was much less efficacious. Recombinant transforming growth factor β added at a dose similar to that found in the colostrum preparation (12.5 ng/rat), reduced injury by about 60%. Addition of colostrum to drinking water (10% vol/vol) prevented villus shortening in the mouse model of small intestinal injury. Addition of milk preparation was ineffective. Colostrum increased proliferation and cell migration of RIE-1 and HT-29 cells. These effects were mainly due to constituents of the colostrum with molecular weights greater than 30kDa. CONCLUSIONS—Bovine colostrum could provide a novel, inexpensive approach for the prevention and treatment of the injurious effects of NSAIDs on the gut and may also be of value for the treatment of other ulcerative conditions of the bowel.
Keywords: gastrointestinal tract; intestinal injury; repair; nutrition
Full Text
The Full Text of this article is available as a PDF (138.0 KB).
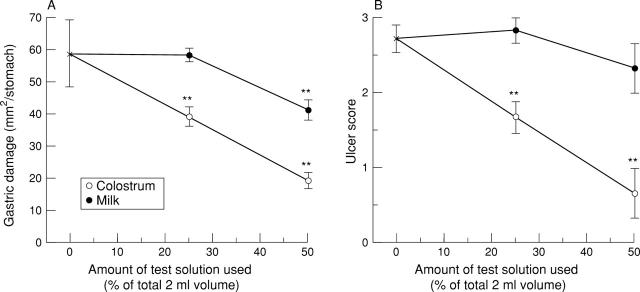
Figure 1 .
Effect of gavage with defatted colostrum and milk on the degree of gastric injury caused by indomethacin. The degree of injury was assessed macroscopically (A), expressed as mm2/stomach and microscopically (B), where microscopic injury was graded with a score from 0 to 4. Results expressed as mean (SEM) of six rats per group. **p<0.01 versus amount of injury seen in animals receiving control solution.
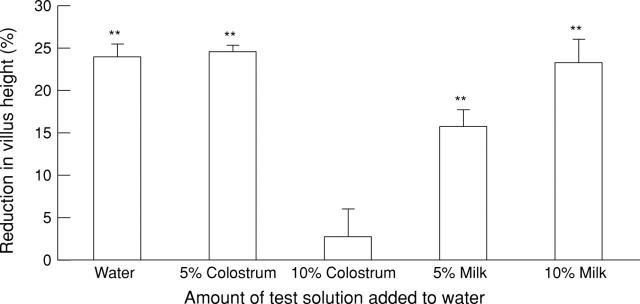
Figure 2 .
Effect of defatted colostrum and milk on the degree of small intestinal injury caused by indomethacin. Data are expressed as the percentage reduction in villus height of indomethacin treated animals compared with animals given the equivalent supplementation but no indomethacin. Results shown are those obtained from the jejunum and are expressed as mean (SD). **p<0.01 versus villus height in animals given the equivalent supplementation but no indomethacin.
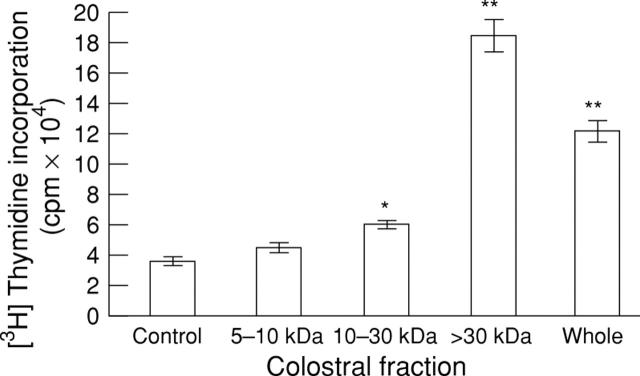
Figure 3 .
Effect of defatted colostrum on cell proliferation in RIE-1 cells. Results expressed as mean (SEM). *p<0.05, **p<0.01 versus cells grown without colostrum.
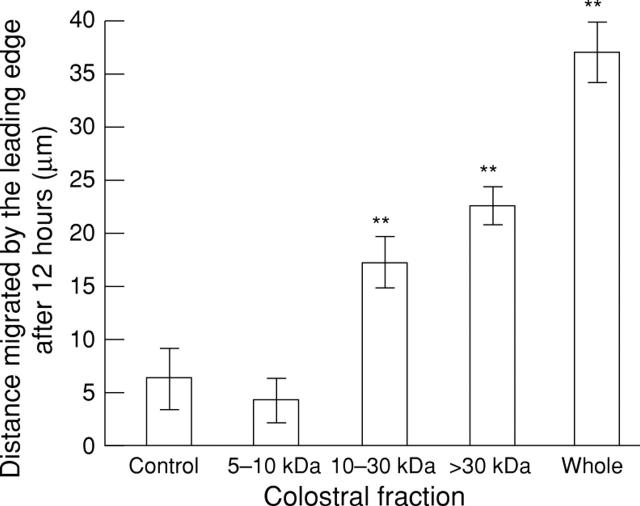
Figure 4 .
Effect of defatted colostrum on HT-29 cell migration, used as an in vitro model of wound repair. **p<0.01 versus distance travelled by cells grown in control solution. Values expressed as mean (SEM).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. C., Howatson A. G., Torrance C. J., Lee F. D., Russell R. I. Gastrointestinal damage associated with the use of nonsteroidal antiinflammatory drugs. N Engl J Med. 1992 Sep 10;327(11):749–754. doi: 10.1056/NEJM199209103271101. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Zanelli G., Smith T., Prouse P., Williams P., Smethurst P., Delacey G., Gumpel M. J., Levi A. J. Nonsteroidal antiinflammatory drug-induced intestinal inflammation in humans. Gastroenterology. 1987 Sep;93(3):480–489. doi: 10.1016/0016-5085(87)90909-7. [DOI] [PubMed] [Google Scholar]
- Chinery R., Playford R. J. Combined intestinal trefoil factor and epidermal growth factor is prophylactic against indomethacin-induced gastric damage in the rat. Clin Sci (Lond) 1995 Apr;88(4):401–403. doi: 10.1042/cs0880401. [DOI] [PubMed] [Google Scholar]
- Dignass A. U., Podolsky D. K. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor beta. Gastroenterology. 1993 Nov;105(5):1323–1332. doi: 10.1016/0016-5085(93)90136-z. [DOI] [PubMed] [Google Scholar]
- Distel M., Mueller C., Bluhmki E., Fries J. Safety of meloxicam: a global analysis of clinical trials. Br J Rheumatol. 1996 Apr;35 (Suppl 1):68–77. doi: 10.1093/rheumatology/35.suppl_1.68. [DOI] [PubMed] [Google Scholar]
- Fries J. F. NSAID gastropathy: the second most deadly rheumatic disease? Epidemiology and risk appraisal. J Rheumatol Suppl. 1991 Mar;28:6–10. [PubMed] [Google Scholar]
- Langman M. J., Morgan L., Worrall A. Use of anti-inflammatory drugs by patients admitted with small or large bowel perforations and haemorrhage. Br Med J (Clin Res Ed) 1985 Feb 2;290(6465):347–349. doi: 10.1136/bmj.290.6465.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S., Shaw-Smith C. Non-steroidal anti-inflammatory drugs: how do they damage the gut? Br J Rheumatol. 1994 Jul;33(7):605–612. doi: 10.1093/rheumatology/33.7.605. [DOI] [PubMed] [Google Scholar]
- MacDonald T. M., Morant S. V., Robinson G. C., Shield M. J., McGilchrist M. M., Murray F. E., McDevitt D. G. Association of upper gastrointestinal toxicity of non-steroidal anti-inflammatory drugs with continued exposure: cohort study. BMJ. 1997 Nov 22;315(7119):1333–1337. doi: 10.1136/bmj.315.7119.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastuszak A. L., Schüler L., Speck-Martins C. E., Coelho K. E., Cordello S. M., Vargas F., Brunoni D., Schwarz I. V., Larrandaburu M., Safattle H. Use of misoprostol during pregnancy and Möbius' syndrome in infants. N Engl J Med. 1998 Jun 25;338(26):1881–1885. doi: 10.1056/NEJM199806253382604. [DOI] [PubMed] [Google Scholar]
- Playford R. J., Marchbank T., Chinery R., Evison R., Pignatelli M., Boulton R. A., Thim L., Hanby A. M. Human spasmolytic polypeptide is a cytoprotective agent that stimulates cell migration. Gastroenterology. 1995 Jan;108(1):108–116. doi: 10.1016/0016-5085(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Playford R. J., Marchbank T., Goodlad R. A., Chinery R. A., Poulsom R., Hanby A. M. Transgenic mice that overexpress the human trefoil peptide pS2 have an increased resistance to intestinal damage. Proc Natl Acad Sci U S A. 1996 Mar 5;93(5):2137–2142. doi: 10.1073/pnas.93.5.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford R. J., Vesey D. A., Haldane S., Alison M. R., Calam J. Dose-dependent effects of fentanyl on indomethacin-induced gastric damage. Digestion. 1991;49(4):198–203. doi: 10.1159/000200722. [DOI] [PubMed] [Google Scholar]
- Playford R. J., Woodman A. C., Clark P., Watanapa P., Vesey D., Deprez P. H., Williamson R. C., Calam J. Effect of luminal growth factor preservation on intestinal growth. Lancet. 1993 Apr 3;341(8849):843–848. doi: 10.1016/0140-6736(93)93057-8. [DOI] [PubMed] [Google Scholar]
- Rogers M. L., Goddard C., Regester G. O., Ballard F. J., Belford D. A. Transforming growth factor beta in bovine milk: concentration, stability and molecular mass forms. J Endocrinol. 1996 Oct;151(1):77–86. doi: 10.1677/joe.0.1510077. [DOI] [PubMed] [Google Scholar]
- Shing Y. W., Klagsbrun M. Human and bovine milk contain different sets of growth factors. Endocrinology. 1984 Jul;115(1):273–282. doi: 10.1210/endo-115-1-273. [DOI] [PubMed] [Google Scholar]
- Shing Y., Klagsbrun M. Purification and characterization of a bovine colostrum-derived growth factor. Mol Endocrinol. 1987 May;1(5):335–338. doi: 10.1210/mend-1-5-335. [DOI] [PubMed] [Google Scholar]
- Simmen F. A., Cera K. R., Mahan D. C. Stimulation by colostrum or mature milk of gastrointestinal tissue development in newborn pigs. J Anim Sci. 1990 Nov;68(11):3596–3603. doi: 10.2527/1990.68113596x. [DOI] [PubMed] [Google Scholar]
- Steimer K. S., Packard R., Holden D., Klagsbrun M. The serum-free growth of cultured cells in bovine colostrum and in milk obtained later in the lactation period. J Cell Physiol. 1981 Nov;109(2):223–234. doi: 10.1002/jcp.1041090205. [DOI] [PubMed] [Google Scholar]
- Svanes K., Ito S., Takeuchi K., Silen W. Restitution of the surface epithelium of the in vitro frog gastric mucosa after damage with hyperosmolar sodium chloride. Morphologic and physiologic characteristics. Gastroenterology. 1982 Jun;82(6):1409–1426. [PubMed] [Google Scholar]
- Wojtulewski J. A., Schattenkirchner M., Barceló P., Le Loët X., Bevis P. J., Bluhmki E., Distel M. A six-month double-blind trial to compare the efficacy and safety of meloxicam 7.5 mg daily and naproxen 750 mg daily in patients with rheumatoid arthritis. Br J Rheumatol. 1996 Apr;35 (Suppl 1):22–28. doi: 10.1093/rheumatology/35.suppl_1.22. [DOI] [PubMed] [Google Scholar]
- Xanthou M., Bines J., Walker W. A. Human milk and intestinal host defense in newborns: an update. Adv Pediatr. 1995;42:171–208. [PubMed] [Google Scholar]
- Xu R. J. Development of the newborn GI tract and its relation to colostrum/milk intake: a review. Reprod Fertil Dev. 1996;8(1):35–48. doi: 10.1071/rd9960035. [DOI] [PubMed] [Google Scholar]