Abstract
BACKGROUND—The trefoil peptides are major secretory products of mucus cells of the gastrointestinal tract and show increased expression after inflammatory or ulcerative damage. Recombinant human TFF2 (spasmolytic polypeptide) has been shown to be cytoprotective, and enhances repair in models of gastric injury. AIMS—To test the healing effects of recombinant human (h)TFF2 in a rat model of chronic colitis. METHODS—Colitis was induced by intracolonic administration of dinitrobenzene sulphonic acid in ethanol. Mucosal repair was quantified macroscopically, microscopically by image analysis of tissue histology, and by measuring myeloperoxidase activity. RESULTS—Initial validation studies showed that maximal injury and inflammation occurred at the end of the first week after colitis induction (active phase), and that spontaneous healing was complete by eight weeks. Once daily intrarectal application of hTFF2 (2.5 mg/kg; approximately 0.5 mg/rat) for five days after maximal damage had been sustained, reduced both microscopic and macroscopic injury by 80% and inflammatory index by 50% compared with vehicle controls. In addition, endogenous concentrations of rat TFF2 and TFF3 (intestinal trefoil factor) were increased in the active phase of colitis and were reduced to basal levels by hTFF2 treatment. CONCLUSIONS—This study has shown that hTFF2 enhances the rate of colonic epithelial repair, and reduces local inflammation in a rat model of colitis, and suggests that luminal application of trefoil peptides may have therapeutic potential in the treatment of inflammatory bowel disease.
Keywords: trefoil peptide; TFF2; spasmolytic polypeptide; colitis; repair
Full Text
The Full Text of this article is available as a PDF (162.5 KB).
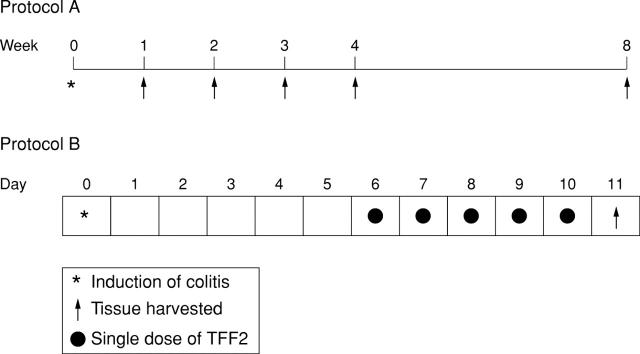
Figure 1 .
Schematic representation of the time course of DNBS/ethanol induced colitis and resolution (protocol A), and the TFF2 healing study (protocol B).
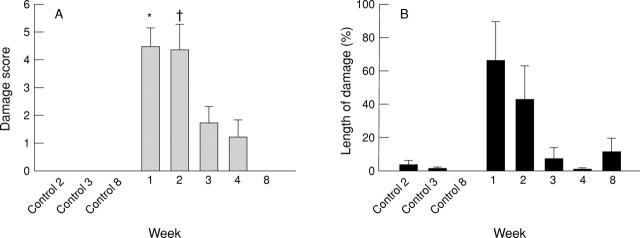
Figure 2 .
Macroscopic (A; graded point scale) and microscopic (B; ratio of the length of epithelial damage to total section length) damage quantitation in control rats or rats with colitis. *p=0.006, †p<0.02.
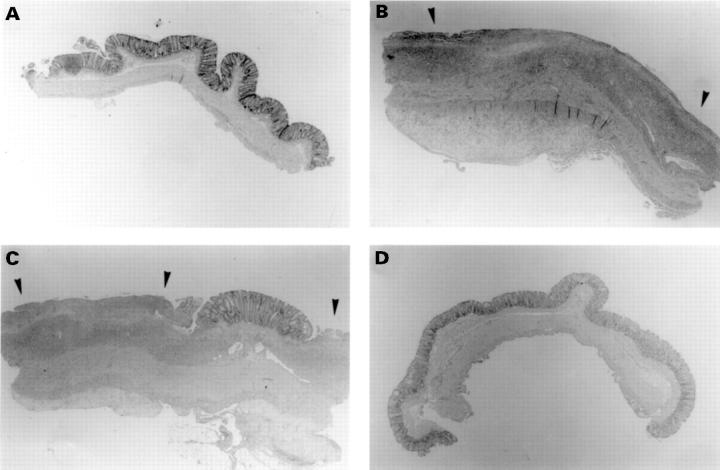
Figure 3 .
Haematoxylin and eosin stained histological sections of rat colonic mucosa from the time course study. (A) Saline control, (B) one week, (C) two weeks, (D) eight weeks after colitis induction. Ulceration is indicated by arrows. Original magnification × 16.
Figure 4 .
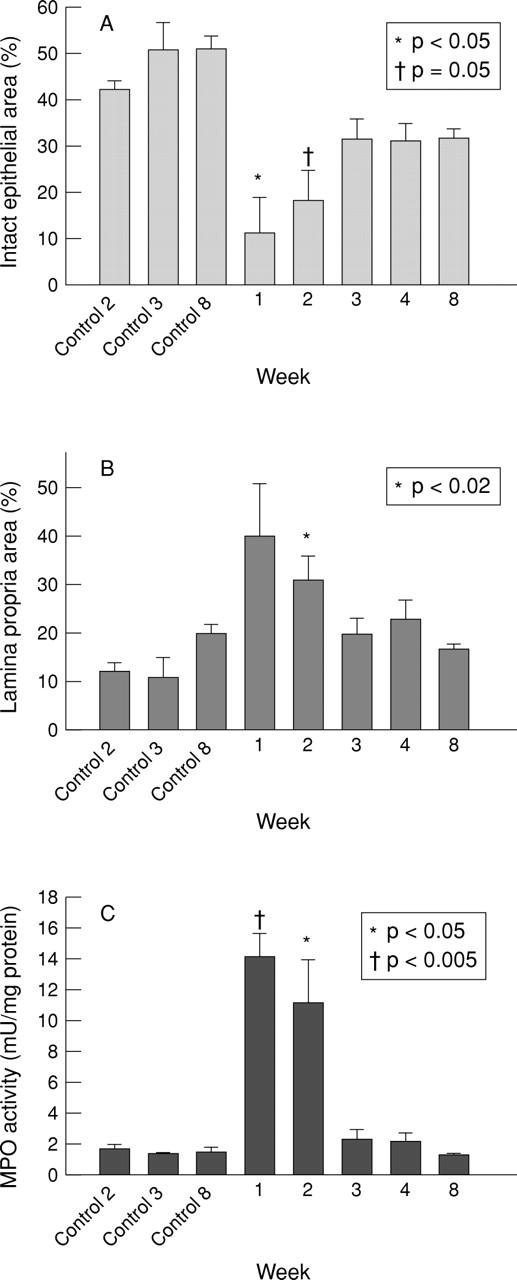
Time course quantitation of the microscopic cross sectional area of mucosal compartment damage after DNBS/ethanol colitis induction, expressed as a percentage of the total cross sectional area of histological sections of epithelium (A) and lamina propria (B); (C) myeloperoxidase (MPO) activity after DNBS/ethanol colitis.
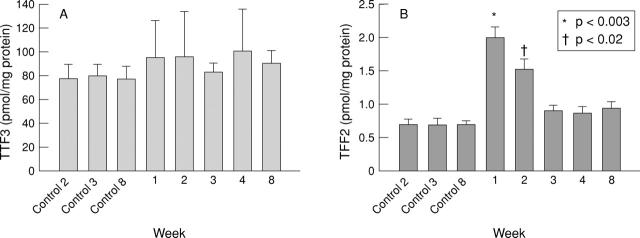
Figure 5 .
Tissue concentrations of the endogenous trefoil peptides TFF3 (A) and TFF2 (B) after induction of colitis by DNBS/ethanol.
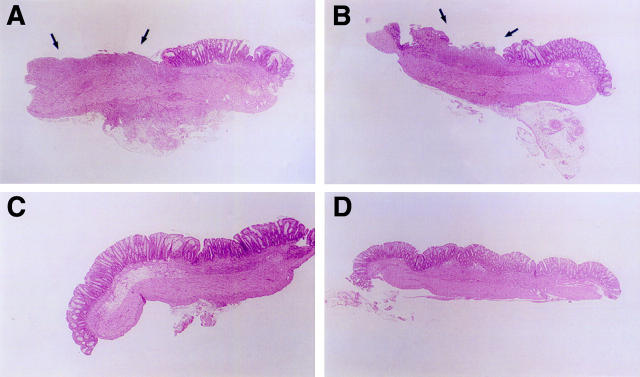
Figure 6 .
Histological sections of rat colonic mucosa six days after DNBS/ethanol colitis induction and treatment for five days with methylcellulose (A,B) or TFF2 (C,D). Sections stained with haematoxylin and eosin. Areas of erosion and gross inflammatory cell infiltrate are arrowed. Original magnification × 16.
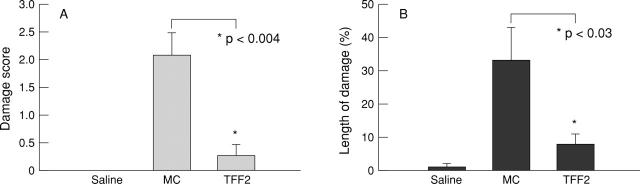
Figure 7 .
Quantitation of (A) macroscopic colitis score and (B) microscopic cellular damage expressed as a ratio of the length of epithelial damage to total histological section length after DNBS/ethanol colitis and treatment with vehicle or TFF2. MC, methylcellulose.
Figure 8 .
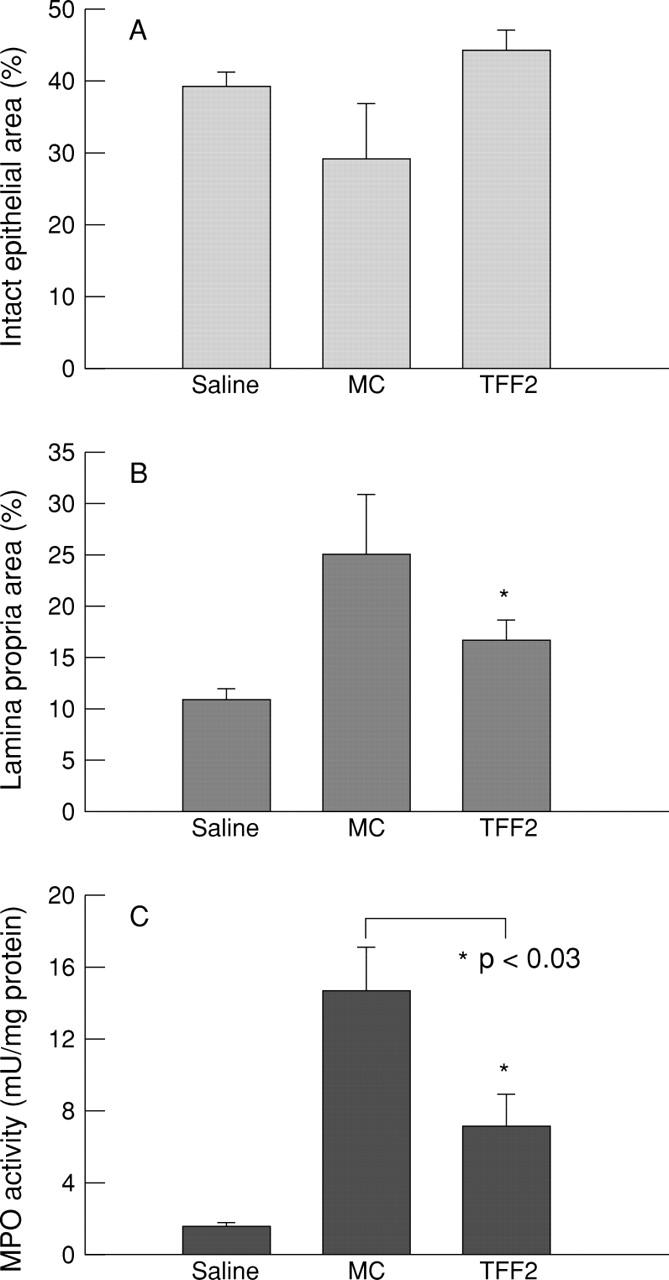
Effect of vehicle (methylcellulose; MC) or TFF2 on the microscopic cross sectional area of mucosal compartment damage after DNBS/ethanol colitis induction, expressed as a percentage of the total cross sectional area of histological sections of epithelium (A) or lamina propria (B); (C) mucosal myeloperoxidase (MPO) activity.
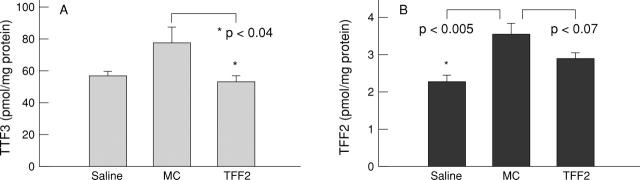
Figure 9 .
Effect of vehicle (methylcellulose; MC) or TFF2 on endogenous mucosal TFF2 and TFF3 concentrations after DNBS/ethanol colitis induction.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alison M. R., Chinery R., Poulsom R., Ashwood P., Longcroft J. M., Wright N. A. Experimental ulceration leads to sequential expression of spasmolytic polypeptide, intestinal trefoil factor, epidermal growth factor and transforming growth factor alpha mRNAs in rat stomach. J Pathol. 1995 Apr;175(4):405–414. doi: 10.1002/path.1711750408. [DOI] [PubMed] [Google Scholar]
- Appleyard C. B., Wallace J. L. Reactivation of hapten-induced colitis and its prevention by anti-inflammatory drugs. Am J Physiol. 1995 Jul;269(1 Pt 1):G119–G125. doi: 10.1152/ajpgi.1995.269.1.G119. [DOI] [PubMed] [Google Scholar]
- Babyatsky M. W., deBeaumont M., Thim L., Podolsky D. K. Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology. 1996 Feb;110(2):489–497. doi: 10.1053/gast.1996.v110.pm8566596. [DOI] [PubMed] [Google Scholar]
- Boughton-Smith N. K., Wallace J. L., Morris G. P., Whittle B. J. The effect of anti-inflammatory drugs on eicosanoid formation in a chronic model of inflammatory bowel disease in the rat. Br J Pharmacol. 1988 May;94(1):65–72. doi: 10.1111/j.1476-5381.1988.tb11500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bradley P. P., Priebat D. A., Christensen R. D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982 Mar;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Cook G. A., Thim L., Yeomans N. D., Giraud A. S. Oral human spasmolytic polypeptide protects against aspirin-induced gastric injury in rats. J Gastroenterol Hepatol. 1998 Apr;13(4):363–370. doi: 10.1111/j.1440-1746.1998.tb00647.x. [DOI] [PubMed] [Google Scholar]
- Cook G. A., Yeomans N. D., Giraud A. S. Temporal expression of trefoil peptides in the TGF-alpha knockout mouse after gastric ulceration. Am J Physiol. 1997 Jun;272(6 Pt 1):G1540–G1549. doi: 10.1152/ajpgi.1997.272.6.G1540. [DOI] [PubMed] [Google Scholar]
- Dignass A., Lynch-Devaney K., Kindon H., Thim L., Podolsky D. K. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994 Jul;94(1):376–383. doi: 10.1172/JCI117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanby A. M., Poulsom R., Singh S., Elia G., Jeffery R. E., Wright N. A. Spasmolytic polypeptide is a major antral peptide: distribution of the trefoil peptides human spasmolytic polypeptide and pS2 in the stomach. Gastroenterology. 1993 Oct;105(4):1110–1116. doi: 10.1016/0016-5085(93)90956-d. [DOI] [PubMed] [Google Scholar]
- Hauser F., Hoffmann W. xP1 and xP4. P-domain peptides expressed in Xenopus laevis stomach mucosa. J Biol Chem. 1991 Nov 5;266(31):21306–21309. [PubMed] [Google Scholar]
- Hauser F., Roeben C., Hoffmann W. xP2, a new member of the P-domain peptide family of potential growth factors, is synthesized in Xenopus laevis skin. J Biol Chem. 1992 Jul 15;267(20):14451–14455. [PubMed] [Google Scholar]
- Itoh H., Tomita M., Uchino H., Kobayashi T., Kataoka H., Sekiya R., Nawa Y. cDNA cloning of rat pS2 peptide and expression of trefoil peptides in acetic acid-induced colitis. Biochem J. 1996 Sep 15;318(Pt 3):939–944. doi: 10.1042/bj3180939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey G. P., Oates P. S., Wang T. C., Babyatsky M. W., Brand S. J. Spasmolytic polypeptide: a trefoil peptide secreted by rat gastric mucous cells. Gastroenterology. 1994 Feb;106(2):336–345. doi: 10.1016/0016-5085(94)90590-8. [DOI] [PubMed] [Google Scholar]
- Kindon H., Pothoulakis C., Thim L., Lynch-Devaney K., Podolsky D. K. Trefoil peptide protection of intestinal epithelial barrier function: cooperative interaction with mucin glycoprotein. Gastroenterology. 1995 Aug;109(2):516–523. doi: 10.1016/0016-5085(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Lefebvre O., Chenard M. P., Masson R., Linares J., Dierich A., LeMeur M., Wendling C., Tomasetto C., Chambon P., Rio M. C. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science. 1996 Oct 11;274(5285):259–262. doi: 10.1126/science.274.5285.259. [DOI] [PubMed] [Google Scholar]
- Mashimo H., Wu D. C., Podolsky D. K., Fishman M. C. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996 Oct 11;274(5285):262–265. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- Masiakowski P., Breathnach R., Bloch J., Gannon F., Krust A., Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982 Dec 20;10(24):7895–7903. doi: 10.1093/nar/10.24.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie C., Marchbank T., Playford R. J., Otto W., Thim L., Parsons M. E. Pancreatic spasmolytic polypeptide protects the gastric mucosa but does not inhibit acid secretion or motility. Am J Physiol. 1997 Jul;273(1 Pt 1):G112–G117. doi: 10.1152/ajpgi.1997.273.1.G112. [DOI] [PubMed] [Google Scholar]
- Morris G. P., Beck P. L., Herridge M. S., Depew W. T., Szewczuk M. R., Wallace J. L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989 Mar;96(3):795–803. [PubMed] [Google Scholar]
- Ogata H., Podolsky D. K. Trefoil peptide expression and secretion is regulated by neuropeptides and acetylcholine. Am J Physiol. 1997 Aug;273(2 Pt 1):G348–G354. doi: 10.1152/ajpgi.1997.273.2.G348. [DOI] [PubMed] [Google Scholar]
- Playford R. J., Marchbank T., Chinery R., Evison R., Pignatelli M., Boulton R. A., Thim L., Hanby A. M. Human spasmolytic polypeptide is a cytoprotective agent that stimulates cell migration. Gastroenterology. 1995 Jan;108(1):108–116. doi: 10.1016/0016-5085(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Lynch-Devaney K., Stow J. L., Oates P., Murgue B., DeBeaumont M., Sands B. E., Mahida Y. R. Identification of human intestinal trefoil factor. Goblet cell-specific expression of a peptide targeted for apical secretion. J Biol Chem. 1993 Mar 25;268(9):6694–6702. [PubMed] [Google Scholar]
- Poulsom R. Trefoil peptides. Baillieres Clin Gastroenterol. 1996 Mar;10(1):113–134. doi: 10.1016/s0950-3528(96)90043-3. [DOI] [PubMed] [Google Scholar]
- Procaccino F., Reinshagen M., Hoffmann P., Zeeh J. M., Lakshmanan J., McRoberts J. A., Patel A., French S., Eysselein V. E. Protective effect of epidermal growth factor in an experimental model of colitis in rats. Gastroenterology. 1994 Jul;107(1):12–17. doi: 10.1016/0016-5085(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Bellocq J. P., Daniel J. Y., Tomasetto C., Lathe R., Chenard M. P., Batzenschlager A., Chambon P. Breast cancer-associated pS2 protein: synthesis and secretion by normal stomach mucosa. Science. 1988 Aug 5;241(4866):705–708. doi: 10.1126/science.3041593. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Chenard M. P., Wolf C., Marcellin L., Tomasetto C., Lathe R., Bellocq J. P., Chambon P. Induction of pS2 and hSP genes as markers of mucosal ulceration of the digestive tract. Gastroenterology. 1991 Feb;100(2):375–379. doi: 10.1016/0016-5085(91)90205-y. [DOI] [PubMed] [Google Scholar]
- Sands B. E., Podolsky D. K. The trefoil peptide family. Annu Rev Physiol. 1996;58:253–273. doi: 10.1146/annurev.ph.58.030196.001345. [DOI] [PubMed] [Google Scholar]
- Suemori S., Lynch-Devaney K., Podolsky D. K. Identification and characterization of rat intestinal trefoil factor: tissue- and cell-specific member of the trefoil protein family. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11017–11021. doi: 10.1073/pnas.88.24.11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thim L. A new family of growth factor-like peptides. 'Trefoil' disulphide loop structures as a common feature in breast cancer associated peptide (pS2), pancreatic spasmolytic polypeptide (PSP), and frog skin peptides (spasmolysins). FEBS Lett. 1989 Jun 19;250(1):85–90. doi: 10.1016/0014-5793(89)80690-8. [DOI] [PubMed] [Google Scholar]
- Thim L., Norris K., Norris F., Nielsen P. F., Bjørn S. E., Christensen M., Petersen J. Purification and characterization of the trefoil peptide human spasmolytic polypeptide (hSP) produced in yeast. FEBS Lett. 1993 Mar 8;318(3):345–352. doi: 10.1016/0014-5793(93)80543-4. [DOI] [PubMed] [Google Scholar]
- Thim L. Trefoil peptides: from structure to function. Cell Mol Life Sci. 1997 Dec;53(11-12):888–903. doi: 10.1007/s000180050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M., Itoh H., Ishikawa N., Higa A., Ide H., Murakumo Y., Maruyama H., Koga Y., Nawa Y. Molecular cloning of mouse intestinal trefoil factor and its expression during goblet cell changes. Biochem J. 1995 Oct 1;311(Pt 1):293–297. doi: 10.1042/bj3110293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L., Keenan C. M. An orally active inhibitor of leukotriene synthesis accelerates healing in a rat model of colitis. Am J Physiol. 1990 Apr;258(4 Pt 1):G527–G534. doi: 10.1152/ajpgi.1990.258.4.G527. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Le T., Carter L., Appleyard C. B., Beck P. L. Hapten-induced chronic colitis in the rat: alternatives to trinitrobenzene sulfonic acid. J Pharmacol Toxicol Methods. 1995 Aug;33(4):237–239. doi: 10.1016/1056-8719(95)00001-x. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Poulsom R., Stamp G. W., Hall P. A., Jeffery R. E., Longcroft J. M., Rio M. C., Tomasetto C., Chambon P. Epidermal growth factor (EGF/URO) induces expression of regulatory peptides in damaged human gastrointestinal tissues. J Pathol. 1990 Dec;162(4):279–284. doi: 10.1002/path.1711620402. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Poulsom R., Stamp G., Van Noorden S., Sarraf C., Elia G., Ahnen D., Jeffery R., Longcroft J., Pike C. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Gastroenterology. 1993 Jan;104(1):12–20. doi: 10.1016/0016-5085(93)90830-6. [DOI] [PubMed] [Google Scholar]