Abstract
BACKGROUND—Dietary lectins can alter the proliferation of colonic cells. Differentiation is regulated by adhesion molecules which, being glycosylated, are targets for lectin binding. AIMS—To examine the effects of dietary lectins on differentiation, adhesion, and proliferation of colorectal cancer cells. METHODS—Differentiation was assessed in three dimensional gels, adhesion by aggregation assay, and proliferation by 3H thymidine incorporation. The role of the epithelial cell adhesion molecule (epCAM) was studied using a specific monoclonal antibody in blocking studies and Western blots. The human colon cancer cell lines LS174T, SW1222, and HT29 were studied. RESULTS—The cell line LS174T differentiated in the presence of Vicia faba agglutinin (VFA) into gland like structures. This was inhibited by anti-epCAM monoclonal antibody. Expression of epCAM itself was unaffected. VFA as well as wheat germ agglutinin (WGA) and the edible mushroom lectin (Agaricus bisporus lectin, ABL) significantly aggregated LS174T cells but peanut agglutinin (PNA) and soybean agglutinin (SBA) did not. All lectins aggregated SW1222 and HT29 cells. Aggregation was blocked by the corresponding sugars. Aggregation of cells by VFA was also inhibited by anti-epCAM. VFA, ABL, and WGL inhibited proliferation of all the cell lines; PNA stimulated proliferation of HT29 and SW1222 cells. In competition studies all sugars blocked aggregation and proliferation of all cell lines, except that the addition of mannose alone inhibited proliferation. CONCLUSION—VFA stimulated an undifferentiated colon cancer cell line to differentiate into gland like structures. The adhesion molecule epCAM is involved in this. Dietary or therapeutic VFA may slow progression of colon cancer.
Keywords: lectins; differentiation; proliferation; polarity; colon cancer
Full Text
The Full Text of this article is available as a PDF (154.8 KB).
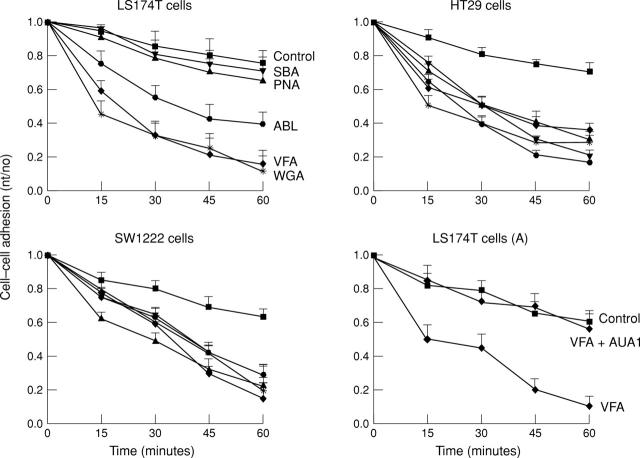
Figure 1 .
Effects of lectins on cell-cell adhesion of LS174T, SW1222, and HT29 cells. Data represent the mean (SD) of at least three experiments.
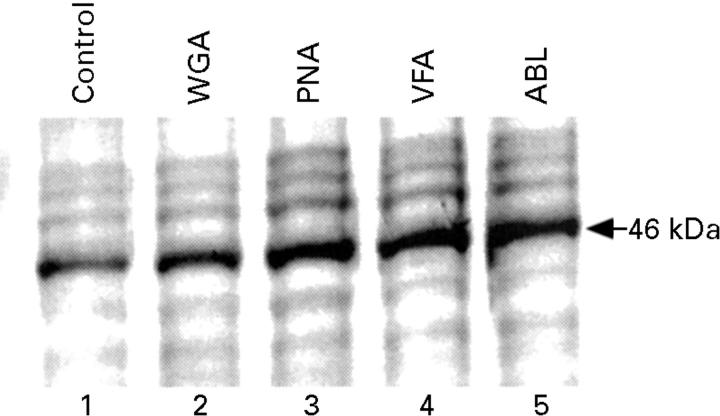
Figure 2 .
Effect of lectins on the expression of epCAM in the LS174T cells. Cells were serum starved overnight, then fed with fresh medium alone (lane 1) or supplemented with different lectins (VFA, WGA, PNA, SBA, and ABL at 10 µg/ml), and incubated for 48 hours. Molecular weight marker of 46 kDa is indicated by the arrow.
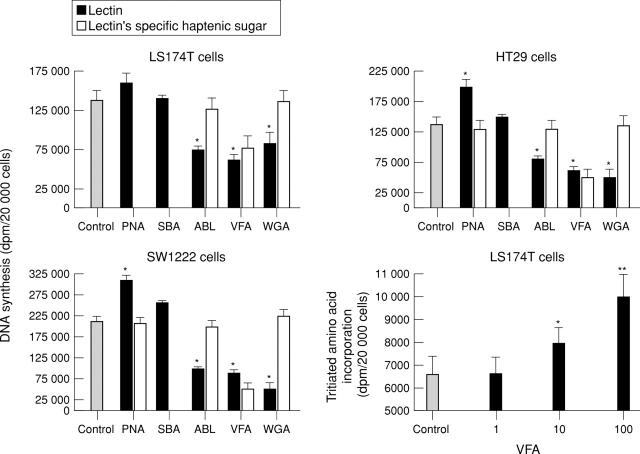
Figure 3 .
Effect of lectins on cell proliferation of LS174T, SW1222, and HT29 cells. Cells were plated in complete medium alone (control) or supplemented with lectins PNA, SBA, ABL, VFA, and WGA (1, 10, or 100 µg/ml); and in the presence of the lectins' specific haptenic sugar (50 mM) for 48 hours. VFA (1, 10, 100 µg/ml) dose dependently increased amino acid incorporation into LS174T cells (B). *p<0.05; **p<0.01. Data represent the means (SD) of triplicate determinations.
Figure 4 .
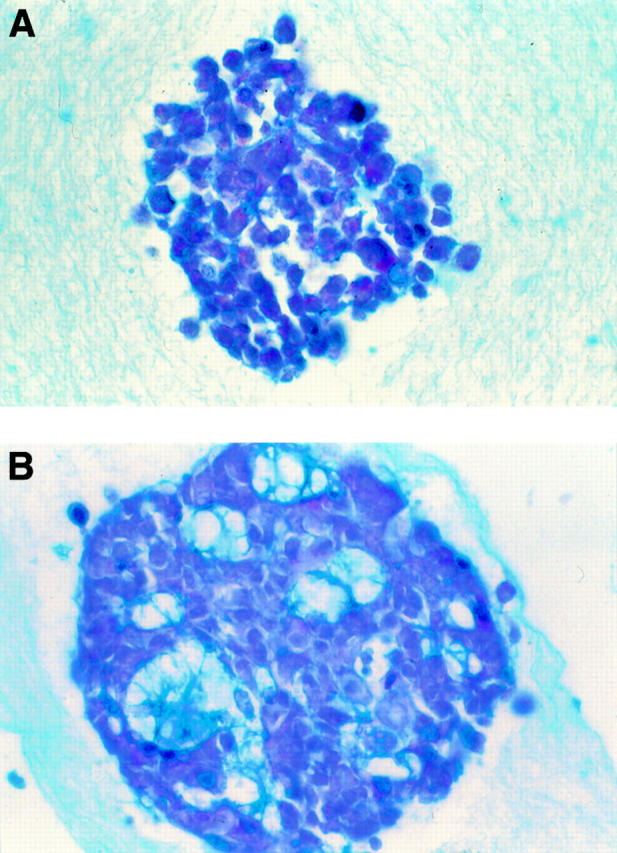
Alcian blue-PAS stained photomicrographs of LS174T cells grown in 3D collagen gels. (A) Control cells grew in amorphous clumps. (B) In the presence of VFA (10 µg/ml) for 14 days the cells grew into gland like structures composed of polarised cells arranged around a central lumen containing mucin.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Boland C. R., Martin M. A., Goldstein I. J. Lectin reactivities as intermediate biomarkers in premalignant colorectal epithelium. J Cell Biochem Suppl. 1992;16G:103–109. doi: 10.1002/jcb.240501119. [DOI] [PubMed] [Google Scholar]
- Brady P. G., Vannier A. M., Banwell J. G. Identification of the dietary lectin, wheat germ agglutinin, in human intestinal contents. Gastroenterology. 1978 Aug;75(2):236–239. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Influence of lectins on the binding of 125I-labeled EGF to human fibroblasts. Biochem Biophys Res Commun. 1977 Nov 21;79(2):545–552. doi: 10.1016/0006-291x(77)90192-9. [DOI] [PubMed] [Google Scholar]
- Chen Y. F., Boland C. R., Kraus E. R., Goldstein I. J. The lectin Griffonia simplicifolia I-A4 (GS I-A4) specifically recognizes terminal alpha-linked N-acetylgalactosaminyl groups and is cytotoxic to the human colon cancer cell lines LS174t and SW1116. Int J Cancer. 1994 May 15;57(4):561–567. doi: 10.1002/ijc.2910570420. [DOI] [PubMed] [Google Scholar]
- Goldman R., Sharon N., Lotan R. A differential response elicited in macrophages on interaction with lectins. Exp Cell Res. 1976 May;99(2):408–422. doi: 10.1016/0014-4827(76)90598-x. [DOI] [PubMed] [Google Scholar]
- Hudson D. L., Sleeman J., Watt F. M. CD44 is the major peanut lectin-binding glycoprotein of human epidermal keratinocytes and plays a role in intercellular adhesion. J Cell Sci. 1995 May;108(Pt 5):1959–1970. doi: 10.1242/jcs.108.5.1959. [DOI] [PubMed] [Google Scholar]
- Jordinson M., Playford R. J., Calam J. Effects of a panel of dietary lectins on cholecystokinin release in rats. Am J Physiol. 1997 Oct;273(4 Pt 1):G946–G950. doi: 10.1152/ajpgi.1997.273.4.G946. [DOI] [PubMed] [Google Scholar]
- Kim M., Rao M. V., Tweardy D. J., Prakash M., Galili U., Gorelik E. Lectin-induced apoptosis of tumour cells. Glycobiology. 1993 Oct;3(5):447–453. doi: 10.1093/glycob/3.5.447. [DOI] [PubMed] [Google Scholar]
- Kiss R., Camby I., Duckworth C., De Decker R., Salmon I., Pasteels J. L., Danguy A., Yeaton P. In vitro influence of Phaseolus vulgaris, Griffonia simplicifolia, concanavalin A, wheat germ, and peanut agglutinins on HCT-15, LoVo, and SW837 human colorectal cancer cell growth. Gut. 1997 Feb;40(2):253–261. doi: 10.1136/gut.40.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koninkx J. F., Hendriks H. G., van Rossum J. M., van den Ingh T. S., Mouwen J. M. Interaction of legume lectins with the cellular metabolism of differentiated Caco-2 cells. Gastroenterology. 1992 May;102(5):1516–1523. doi: 10.1016/0016-5085(92)91709-d. [DOI] [PubMed] [Google Scholar]
- Leibovitz A., Stinson J. C., McCombs W. B., 3rd, McCoy C. E., Mazur K. C., Mabry N. D. Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 1976 Dec;36(12):4562–4569. [PubMed] [Google Scholar]
- Litvinov S. V., Bakker H. A., Gourevitch M. M., Velders M. P., Warnaar S. O. Evidence for a role of the epithelial glycoprotein 40 (Ep-CAM) in epithelial cell-cell adhesion. Cell Adhes Commun. 1994 Oct;2(5):417–428. doi: 10.3109/15419069409004452. [DOI] [PubMed] [Google Scholar]
- Litvinov S. V., Velders M. P., Bakker H. A., Fleuren G. J., Warnaar S. O. Ep-CAM: a human epithelial antigen is a homophilic cell-cell adhesion molecule. J Cell Biol. 1994 Apr;125(2):437–446. doi: 10.1083/jcb.125.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Gagliardi G., Nasim M. M., Alison M. R., Oates T., Lalani E. N., Stamp G. W., Pignatelli M. TGF-alpha can act as morphogen and/or mitogen in a colon-cancer cell line. Int J Cancer. 1994 Feb 15;56(4):603–608. doi: 10.1002/ijc.2910560423. [DOI] [PubMed] [Google Scholar]
- Nachbar M. S., Oppenheim J. D. Lectins in the United States diet: a survey of lectins in commonly consumed foods and a review of the literature. Am J Clin Nutr. 1980 Nov;33(11):2338–2345. doi: 10.1093/ajcn/33.11.2338. [DOI] [PubMed] [Google Scholar]
- Pignatelli M., Liu D., Nasim M. M., Stamp G. W., Hirano S., Takeichi M. Morphoregulatory activities of E-cadherin and beta-1 integrins in colorectal tumour cells. Br J Cancer. 1992 Oct;66(4):629–634. doi: 10.1038/bjc.1992.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter J. D. Risk factors for colon neoplasia--epidemiology and biology. Eur J Cancer. 1995 Jul-Aug;31A(7-8):1033–1038. doi: 10.1016/0959-8049(95)00125-3. [DOI] [PubMed] [Google Scholar]
- Pusztai A., Ewen S. W., Grant G., Peumans W. J., van Damme E. J., Rubio L., Bardocz S. Relationship between survival and binding of plant lectins during small intestinal passage and their effectiveness as growth factors. Digestion. 1990;46 (Suppl 2):308–316. doi: 10.1159/000200402. [DOI] [PubMed] [Google Scholar]
- Rhodes J. M. Unifying hypothesis for inflammatory bowel disease and associated colon cancer: sticking the pieces together with sugar. Lancet. 1996 Jan 6;347(8993):40–44. doi: 10.1016/s0140-6736(96)91563-9. [DOI] [PubMed] [Google Scholar]
- Rogers G. T. Carcinoembryonic antigens and related glycoproteins. Molecular aspects and specificity. Biochim Biophys Acta. 1983 Dec 29;695(3-4):227–249. doi: 10.1016/0304-419x(83)90013-6. [DOI] [PubMed] [Google Scholar]
- Rubio L. A., Grant G., Bardocz S., Dewey P., Pusztai A. Nutritional response of growing rats to faba beans (Vicia faba L., minor) and faba bean fractions. Br J Nutr. 1991 Nov;66(3):533–542. doi: 10.1079/bjn19910053. [DOI] [PubMed] [Google Scholar]
- Rutishauser U., Acheson A., Hall A. K., Mann D. M., Sunshine J. The neural cell adhesion molecule (NCAM) as a regulator of cell-cell interactions. Science. 1988 Apr 1;240(4848):53–57. doi: 10.1126/science.3281256. [DOI] [PubMed] [Google Scholar]
- Rutzky L. P., Kaye C. I., Siciliano M. J., Chao M., Kahan B. D. Longitudinal karyotype and genetic signature analysis of cultured human colon adenocarcinoma cell lines LS180 and LS174T. Cancer Res. 1980 May;40(5):1443–1448. [PubMed] [Google Scholar]
- Ryder S. D., Jacyna M. R., Levi A. J., Rizzi P. M., Rhodes J. M. Peanut ingestion increases rectal proliferation in individuals with mucosal expression of peanut lectin receptor. Gastroenterology. 1998 Jan;114(1):44–49. doi: 10.1016/s0016-5085(98)70631-6. [DOI] [PubMed] [Google Scholar]
- Ryder S. D., Parker N., Ecclestone D., Haqqani M. T., Rhodes J. M. Peanut lectin stimulates proliferation in colonic explants from patients with inflammatory bowel disease and colon polyps. Gastroenterology. 1994 Jan;106(1):117–124. doi: 10.1016/s0016-5085(94)94775-9. [DOI] [PubMed] [Google Scholar]
- Ryder S. D., Smith J. A., Rhodes E. G., Parker N., Rhodes J. M. Proliferative responses of HT29 and Caco2 human colorectal cancer cells to a panel of lectins. Gastroenterology. 1994 Jan;106(1):85–93. doi: 10.1016/s0016-5085(94)94527-6. [DOI] [PubMed] [Google Scholar]
- Ryder S. D., Smith J. A., Rhodes J. M. Peanut lectin: a mitogen for normal human colonic epithelium and human HT29 colorectal cancer cells. J Natl Cancer Inst. 1992 Sep 16;84(18):1410–1416. doi: 10.1093/jnci/84.18.1410. [DOI] [PubMed] [Google Scholar]
- Stoscheck C. M., Soderquist A. M., Carpenter G. Biosynthesis of the epidermal growth factor receptor in cultured human cells. Endocrinology. 1985 Feb;116(2):528–535. doi: 10.1210/endo-116-2-528. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991 Mar 22;251(5000):1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Yu L., Fernig D. G., Smith J. A., Milton J. D., Rhodes J. M. Reversible inhibition of proliferation of epithelial cell lines by Agaricus bisporus (edible mushroom) lectin. Cancer Res. 1993 Oct 1;53(19):4627–4632. [PubMed] [Google Scholar]
- Zeng F. Y., Benguría A., Kafert S., André S., Gabius H. J., Villalobo A. Differential response of the epidermal growth factor receptor tyrosine kinase activity to several plant and mammalian lectins. Mol Cell Biochem. 1995 Jan 26;142(2):117–124. doi: 10.1007/BF00928932. [DOI] [PubMed] [Google Scholar]