Abstract
BACKGROUND—The long term outcome of drug related liver disease is unknown. AIMS—To study the natural history of histologically proved drug induced hepatotoxicity. METHODS—110 patients with liver biopsies coded either as drug induced liver disease or hepatitis/cholestasis of unknown aetiology were identified from hospital records 1978-1996. Review of case notes and histology identified 44 patients with definite drug induced hepatotoxicity. Forty surviving patients were invited to attend a follow up clinic. History, examination, full liver screen, and isotope and ultrasound liver scans were repeated in all patients. Repeat liver biopsies were offered to patients with abnormal liver tests. RESULTS—Presentation at index biopsy was jaundice in 24 patients, abnormal liver tests in 17, and hepatic failure in three. Antibiotics (n=13) and non-steroidal anti-inflammatory drugs (n=11) were the most common drugs implicated. Initial histology showed acute hepatitis in six, chronic hepatitis in 20, and cholestasis in 18. At 1-19 years (median 5 years) follow up, 13/33 (39%) patients had persistent significant abnormalities in their liver blood tests and/or scans. Three of the five repeat liver biopsies performed showed significant abnormalities. Factors predicting persistence or development of chronic liver disease were fibrosis and continued exposure to the drug. CONCLUSIONS—Drugs should be considered in the differential diagnosis of abnormal liver function and/or histology, as failure to withdraw the offending drug is associated with a high risk of persistent liver damage.
Keywords: drugs; chronic active hepatitis; toxic hepatitis; diclofenac
Full Text
The Full Text of this article is available as a PDF (113.0 KB).
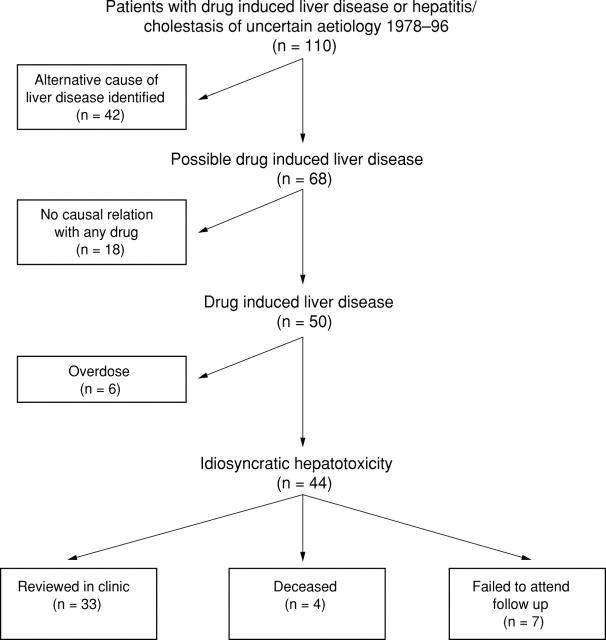
Figure 1 .
Patients with drug induced liver disease or hepatitis/cholestasis of uncertain aetiology, 1978-96; details of patients studied with reasons for exclusion from the study.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almdal T. P., Sørensen T. I. Incidence of parenchymal liver diseases in Denmark, 1981 to 1985: analysis of hospitalization registry data. The Danish Association for the Study of the Liver. Hepatology. 1991 Apr;13(4):650–655. [PubMed] [Google Scholar]
- Banks A. T., Zimmerman H. J., Ishak K. G., Harter J. G. Diclofenac-associated hepatotoxicity: analysis of 180 cases reported to the Food and Drug Administration as adverse reactions. Hepatology. 1995 Sep;22(3):820–827. [PubMed] [Google Scholar]
- Bénichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990 Sep;11(2):272–276. doi: 10.1016/0168-8278(90)90124-a. [DOI] [PubMed] [Google Scholar]
- COOK G. C., SHERLOCK S. JAUNDICE AND ITS RELATION TO THERAPEUTIC AGENTS. Lancet. 1965 Jan 23;1(7378):175–179. doi: 10.1016/s0140-6736(65)90969-4. [DOI] [PubMed] [Google Scholar]
- Danan G. Causality assessment of drug-induced liver injury. Hepatology Working Group. J Hepatol. 1988 Aug;7(1):132–136. doi: 10.1016/s0168-8278(88)80517-8. [DOI] [PubMed] [Google Scholar]
- Døssing M., Andreasen P. B. Drug-induced liver disease in Denmark. An analysis of 572 cases of hepatotoxicity reported to the Danish Board of Adverse Reactions to Drugs. Scand J Gastroenterol. 1982 Mar;17(2):205–211. doi: 10.3109/00365528209182041. [DOI] [PubMed] [Google Scholar]
- Friis H., Andreasen P. B. Drug-induced hepatic injury: an analysis of 1100 cases reported to the Danish Committee on Adverse Drug Reactions between 1978 and 1987. J Intern Med. 1992 Aug;232(2):133–138. doi: 10.1111/j.1365-2796.1992.tb00562.x. [DOI] [PubMed] [Google Scholar]
- Goldstein G. B., Lam K. C., Mistilis S. P. Drug-induced active chronic hepatitis. Am J Dig Dis. 1973 Mar;18(3):177–184. doi: 10.1007/BF01071970. [DOI] [PubMed] [Google Scholar]
- Ishak K. G., Irey N. S. Hepatic injury associated with the phenothiazines. Clinicopathologic and follow-up study of 36 patients. Arch Pathol. 1972 Apr;93(4):283–304. [PubMed] [Google Scholar]
- Iveson T. J., Ryley N. G., Kelly P. M., Trowell J. M., McGee J. O., Chapman R. W. Diclofenac associated hepatitis. J Hepatol. 1990 Jan;10(1):85–89. doi: 10.1016/0168-8278(90)90077-5. [DOI] [PubMed] [Google Scholar]
- Lewis J. H., Zimmerman H. J. Drug-induced liver disease. Med Clin North Am. 1989 Jul;73(4):775–792. doi: 10.1016/s0025-7125(16)30638-1. [DOI] [PubMed] [Google Scholar]
- Lindberg J., Lindholm A., Lundin P., Iwarson S. Trigger factors and HL-A antigens in chronic active hepatitis. Br Med J. 1975 Oct 11;4(5988):77–79. doi: 10.1136/bmj.4.5988.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddrey W. C., Boitnott J. K. Drug-induced chronic liver disease. Gastroenterology. 1977 Jun;72(6):1348–1353. [PubMed] [Google Scholar]
- Maddrey W. C., Boitnott J. K. Isoniazid hepatitis. Ann Intern Med. 1973 Jul;79(1):1–12. doi: 10.7326/0003-4819-79-1-1. [DOI] [PubMed] [Google Scholar]
- Maria V. A., Victorino R. M. Development and validation of a clinical scale for the diagnosis of drug-induced hepatitis. Hepatology. 1997 Sep;26(3):664–669. doi: 10.1002/hep.510260319. [DOI] [PubMed] [Google Scholar]
- Miller R. R. Hospital admissions due to adverse drug reactions. A report from the Boston Collaborative Drug Surveillance Program. Arch Intern Med. 1974 Aug;134(2):219–223. [PubMed] [Google Scholar]
- Mitchell J. R., Jollow D. J., Potter W. Z., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973 Oct;187(1):211–217. [PubMed] [Google Scholar]
- Olsson R., Wiholm B. E., Sand C., Zettergren L., Hultcrantz R., Myrhed M. Liver damage from flucloxacillin, cloxacillin and dicloxacillin. J Hepatol. 1992 May;15(1-2):154–161. doi: 10.1016/0168-8278(92)90029-o. [DOI] [PubMed] [Google Scholar]
- Sallie R. W., McKenzie T., Reed W. D., Quinlan M. F., Shilkin K. B. Diclofenac hepatitis. Aust N Z J Med. 1991 Apr;21(2):251–255. doi: 10.1111/j.1445-5994.1991.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Scully L. J., Clarke D., Barr R. J. Diclofenac induced hepatitis. 3 cases with features of autoimmune chronic active hepatitis. Dig Dis Sci. 1993 Apr;38(4):744–751. doi: 10.1007/BF01316809. [DOI] [PubMed] [Google Scholar]
- Seeff L. B. Drug-induced chronic liver disease, with emphasis on chronic active hepatitis. Semin Liver Dis. 1981 May;1(2):104–115. doi: 10.1055/s-2008-1040723. [DOI] [PubMed] [Google Scholar]
- Sharp J. R., Ishak K. G., Zimmerman H. J. Chronic active hepatitis and severe hepatic necrosis associated with nitrofurantoin. Ann Intern Med. 1980 Jan;92(1):14–19. doi: 10.7326/0003-4819-92-1-14. [DOI] [PubMed] [Google Scholar]
- Toghill P. J., Smith P. G., Benton P., Brown R. C., Matthews H. L. Methyldopa liver damage. Br Med J. 1974 Aug 31;3(5930):545–548. doi: 10.1136/bmj.3.5930.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utili R., Boitnott J. K., Zimmerman H. J. Dantrolene-associated hepatic injury. Incidence and character. Gastroenterology. 1977 Apr;72(4 Pt 1):610–616. [PubMed] [Google Scholar]