Abstract
BACKGROUND/AIMS—The characteristics of pepsinogen screening for gastric cancer were investigated to establish a suitable cut off point for identifying gastric cancer, using endoscopic diagnosis as the yardstick. SUBJECTS/METHODS—Serum pepsinogen concentrations were measured in 5113 subjects who were also screened for gastric cancer by endoscopy. The cut off point for pepsinogen was determined using receiver operator characteristics curves. RESULTS—The most suitable cut off point was a pepsinogen I concentration of less than 70 ng/ml and a ratio of pepsinogen I to pepsinogen II of less than 3.0. Using this cut off point, the sensitivity and specificity of pepsinogen screening for gastric cancer were 84.6% and 73.5% respectively. All cases of gastric cancer in patients with severe atrophic gastritis were detected. However, two of four cases of gastric cancer in patients with mild atrophic gastritis were overlooked. In subjects with mild atrophic gastritis, when gastric cancer arises within the fundic gland region, the size of the lesion determines whether it is possible to detect cancer by serum pepsinogen screening. CONCLUSION—Pepsinogen screening has many advantages, including its suitability for combination with other screening methods because it is simple and inexpensive.
Keywords: pepsinogen; gastric cancer; screening; cut off point; receiver operator characteristics curves; atrophic gastritis
Full Text
The Full Text of this article is available as a PDF (112.7 KB).
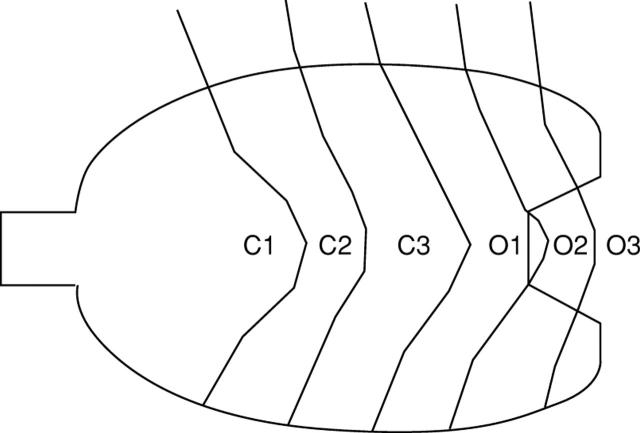
Figure 1 .
Classification of chronic atrophic gastritis according to the location of the border between the fundic and pyloric gland regions by endoscopy: C1, at the angular part of the lesser curvature; C2, in the lower part of the lesser curvature; C3, in the middle part of the lesser curvature; O1, all parts of the lesser curvature are pyloric; O2, the stage between O1 and O3; O3, all mucosa of the stomach is non-acid-secreting.
Figure 2 .
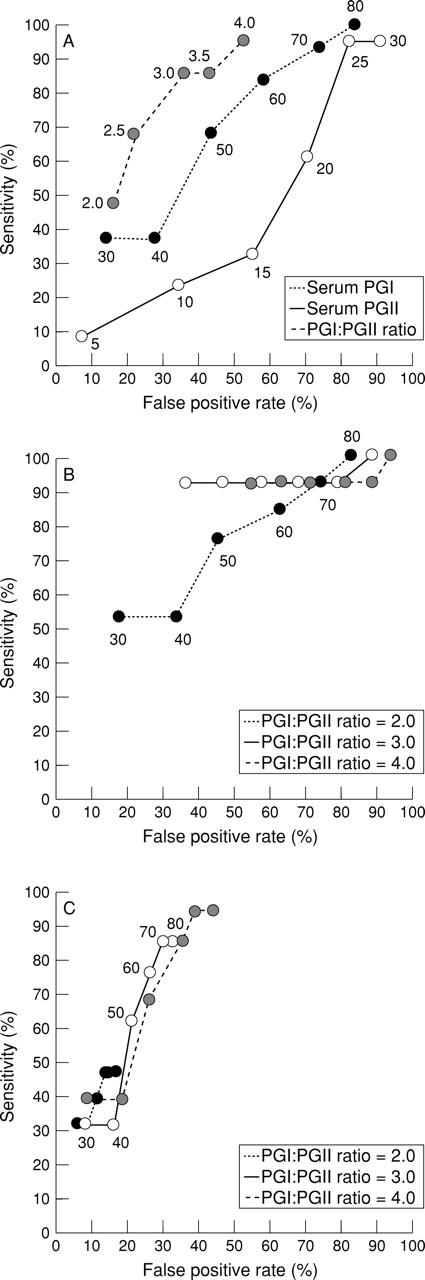
Receiver operator characteristics (ROC) curves generated with serum pepsinogen (PG) I concentrations alone, PG II concentrations alone, and I:II ratios alone (A), various serum PG I concentrations or I:II ratios (B), the combination of various serum PG I concentrations and I:II ratios (C), and by changing the serum PG I concentration from 30 to 80 ng/ml, the PG II concentration from 5 to 30 ng/ml, and the I:II ratio from 2.0 to 4.0 intermittently. The following cut off points are found to be suitable: a I:II ratio of less than 3.0 alone (A), a PG I concentration of less than 30 ng/ml or a I:II ratio of less than 3.0 (B), a PG I concentration of less than 70 ng/ml and a I:II ratio of less than 3.0 (C).
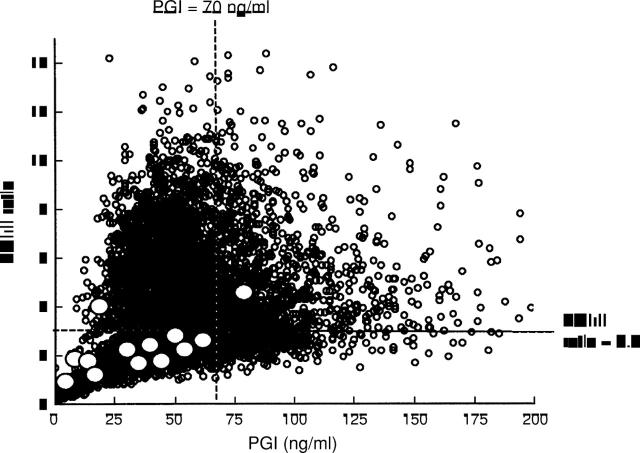
Figure 3 .
Distribution of serum pepsinogen (PG) I concentrations and I:II ratios in all subjects. White circles denote cases of gastric cancer. Of 13 gastric cancer cases, 11 would have been detected by the serum PG method, using a PG I concentration of less than 70 ng/ml and a I:II ratio of less than 3.0 as the cut off point.
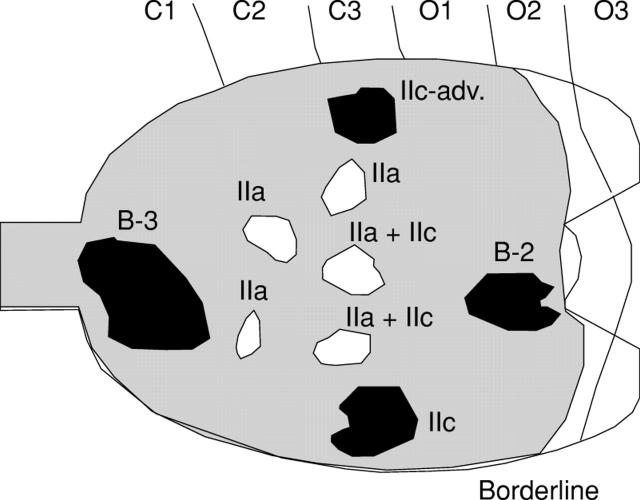
Figure 4 .
Distribution of nine cases of gastric cancer in patients with severe atrophic gastritis. White lesions are differentiated and black are undifferentiated. These nine cases were detected by serum pepsinogen (PG) screening for gastric cancer, using a serum PG I concentration of less than 70 ng/ml and a I:II ratio of less than 3.0 as the cut off point. B-2, Borrmann type 2; B-3, Borrmann type 3; IIc-adv, advanced cancer resembling type IIc. Borderline, border between fundic and pyloric regions.
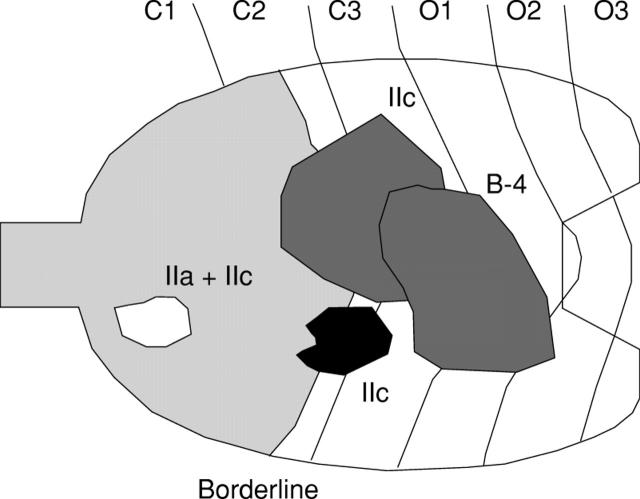
Figure 5 .
Distribution of four cases of gastric cancer in subjects with mild atrophic gastritis. The white lesions are differentiated, and the grey lesions are undifferentiated. Two of the four cases could not be detected by serum pepsinogen (PG) screening using a serum PG I concentration of less than 70 ng/ml and a I:II ratio of less than 3.0 as the cut off point. B-4, Borrmann type 4. Borderline, borderline between fundic and pyloric regions.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fukao A., Hisamichi S., Ohsato N., Fujino N., Endo N., Iha M. Correlation between the prevalence of gastritis and gastric cancer in Japan. Cancer Causes Control. 1993 Jan;4(1):17–20. doi: 10.1007/BF00051709. [DOI] [PubMed] [Google Scholar]
- Fukao A., Tsubono Y., Tsuji I., HIsamichi S., Sugahara N., Takano A. The evaluation of screening for gastric cancer in Miyagi Prefecture, Japan: a population-based case-control study. Int J Cancer. 1995 Jan 3;60(1):45–48. doi: 10.1002/ijc.2910600106. [DOI] [PubMed] [Google Scholar]
- Ichinose M., Miki K., Furihata C., Kageyama T., Hayashi R., Niwa H., Oka H., Matsushima T., Takahashi K. Radioimmunoassay of serum group I and group II pepsinogens in normal controls and patients with various disorders. Clin Chim Acta. 1982 Dec 9;126(2):183–191. doi: 10.1016/0009-8981(82)90034-1. [DOI] [PubMed] [Google Scholar]
- Kodoi A., Yoshihara M., Sumii K., Haruma K., Kajiyama G. Serum pepsinogen in screening for gastric cancer. J Gastroenterol. 1995 Aug;30(4):452–460. doi: 10.1007/BF02347560. [DOI] [PubMed] [Google Scholar]
- Konishi N., Matsumoto K., Hiasa Y., Kitahori Y., Hayashi I., Matsuda H. Tissue and serum pepsinogen I and II in gastric cancer identified using immunohistochemistry and rapid ELISA. J Clin Pathol. 1995 Apr;48(4):364–367. doi: 10.1136/jcp.48.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T., Noda Y., Kumamoto Y., Nishizawa K., Furuya Y., Iwai K., Urahashi S., Kurihara R., Kamata R., Sakata S. [Estimation of frequency, population doses and stochastic risks in stomach mass screening examinations in Japan, 1980]. Nihon Igaku Hoshasen Gakkai Zasshi. 1987 Jul 25;47(7):971–982. [PubMed] [Google Scholar]
- Matsumoto K., Hashimoto K., Samori T., Taniguchi M., Kotera K., Nishi S., Takatsuji M., Higashi K., Itoh K. [Clinical significance of the measurement of serum pepsinogen group I and II by enzyme-linked immunosorbent assay]. Rinsho Byori. 1992 Sep;40(9):977–981. [PubMed] [Google Scholar]
- Miki K., Ichinose M., Ishikawa K. B., Yahagi N., Matsushima M., Kakei N., Tsukada S., Kido M., Ishihama S., Shimizu Y. Clinical application of serum pepsinogen I and II levels for mass screening to detect gastric cancer. Jpn J Cancer Res. 1993 Oct;84(10):1086–1090. doi: 10.1111/j.1349-7006.1993.tb02805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K. [Mass screening of stomach neoplasms by serum pepsinogen analysis]. Nihon Naika Gakkai Zasshi. 1992 May 10;81(5):654–659. [PubMed] [Google Scholar]
- Samloff I. M., Varis K., Ihamaki T., Siurala M., Rotter J. I. Relationships among serum pepsinogen I, serum pepsinogen II, and gastric mucosal histology. A study in relatives of patients with pernicious anemia. Gastroenterology. 1982 Jul;83(1 Pt 2):204–209. [PubMed] [Google Scholar]
- Sitas F., Smallwood R., Jewell D., Millard P. R., Newell D. G., Meuwissen S. G., Moses S., Zwiers A., Forman D. Serum anti-Helicobacter pylori IgG antibodies and pepsinogens A and C as serological markers of chronic atrophic gastritis. Cancer Epidemiol Biomarkers Prev. 1993 Mar-Apr;2(2):119–123. [PubMed] [Google Scholar]
- Stemmermann G. N., Samloff I. M., Nomura A. M., Heilbrun L. K. Serum pepsinogens I and II and stomach cancer. Clin Chim Acta. 1987 Mar 16;163(2):191–198. doi: 10.1016/0009-8981(87)90022-2. [DOI] [PubMed] [Google Scholar]
- Webb P. M., Hengels K. J., Møller H., Newell D. G., Palli D., Elder J. B., Coleman M. P., De Backer G., Forman D. The epidemiology of low serum pepsinogen A levels and an international association with gastric cancer rates. EUROGAST Study Group. Gastroenterology. 1994 Nov;107(5):1335–1344. doi: 10.1016/0016-5085(94)90535-5. [DOI] [PubMed] [Google Scholar]