Abstract
BACKGROUND—Several inflammatory disorders of the intestine are characterised by enhanced expression of tumour necrosis factor α (TNF-α). Monocytes and macrophages have been suggested as a major cellular source of TNF-α in human gut, whereas mast cells, although known to be capable of producing TNF-α, have been poorly examined in this respect. AIMS—To investigate whether human intestinal mast cells can produce TNF-α, and which factors regulate TNF-α production in these cells. METHODS—Mast cells were isolated from surgery tissue specimens of patients undergoing bowel resection because of cancer. Immunohistochemical studies were performed in biopsy specimens derived from 13 patients (two healthy controls, four with Crohn's disease, four with ulcerative colitis, three others). TNF-α mRNA and protein expression were studied in vitro by polymerase chain reaction, RNAse protection assay, western blot, and enzyme linked immunosorbent assay in isolated purified human intestinal mast cells stimulated by IgE receptor crosslinking, intestinal bacteria, and lipopolysaccharide. Cellular localisation of TNF-α was examined by immunohistochemistry. RESULTS—TNF-α mRNA and protein were expressed constitutively in isolated human intestinal mast cells. Expression of TNF-α mRNA and release of TNF-α protein were substantially enhanced by IgE receptor crosslinking and by coculture of mast cells with intestinal bacteria; lipopolysaccharide had only marginal effects. Immunohistochemical studies revealed that approximately 60% of the lamina propria cells with immunoreactivity for TNF-α were mast cells. CONCLUSIONS—The data show that mast cells are an important source of TNF-α in the human intestinal mucosa.
Keywords: tumour necrosis factor; mast cells; inflammatory bowel disease; bacteria
Full Text
The Full Text of this article is available as a PDF (239.0 KB).
Figure 1 .
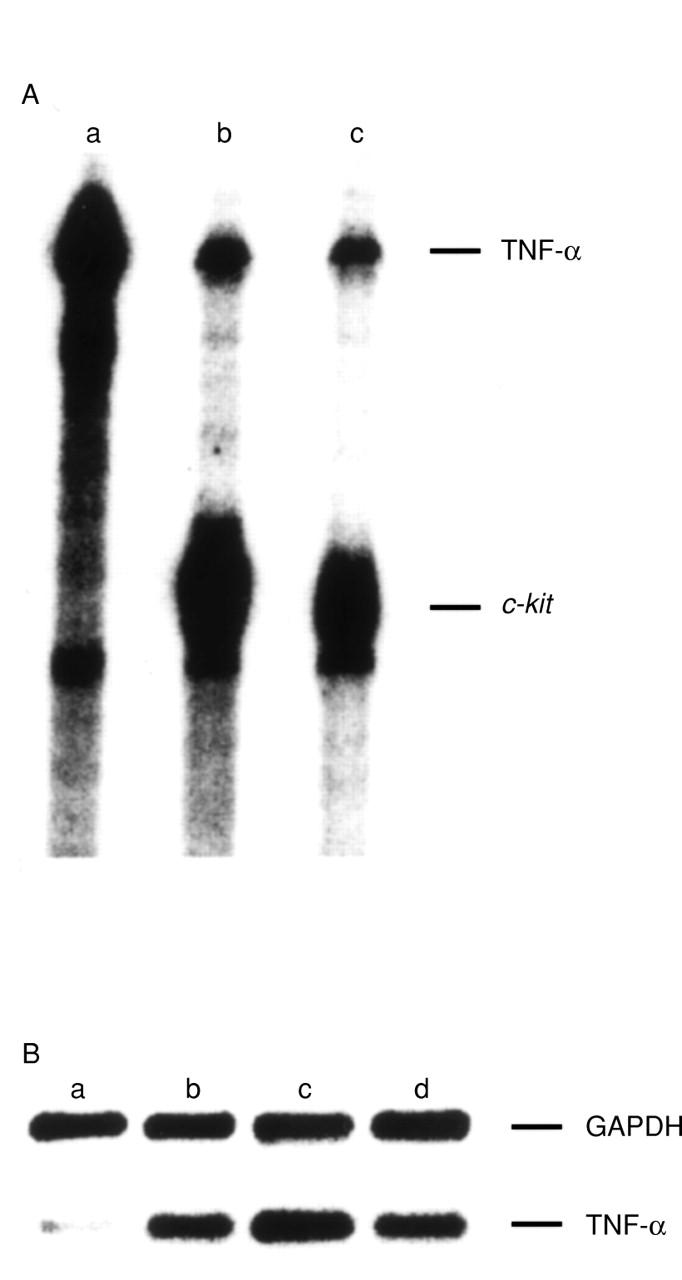
Expression of TNF-α mRNA in purified human intestinal mast cells. (A) RNAse protection assay: 10 µg of total RNA from THP.1 cells stimulated with 300 U/ml IFN γ (a), and 8 µg each of total RNA from unstimulated mast cells (b), and from mast cells stimulated with 100 ng/ml 29C6 (c), respectively (mast cell purity 98%) were hybridised with radiolabelled TNF-α cRNA (a,b,c) and c-kit cRNA (b,c). (B) Primer dropping RT-PCR: total RNA derived from purified mast cells (99% purity) either unstimulated (a), or stimulated with 100 ng/ml 29C6 (b), 4 × 106/ml bacteria (c), or 10 µg/ml LPS (d) was amplified in the presence of primer pairs for TNF-α and (after seven PCR cycles) GAPDH. DNA fragments obtained after 33 cycles are shown.
Figure 2 .
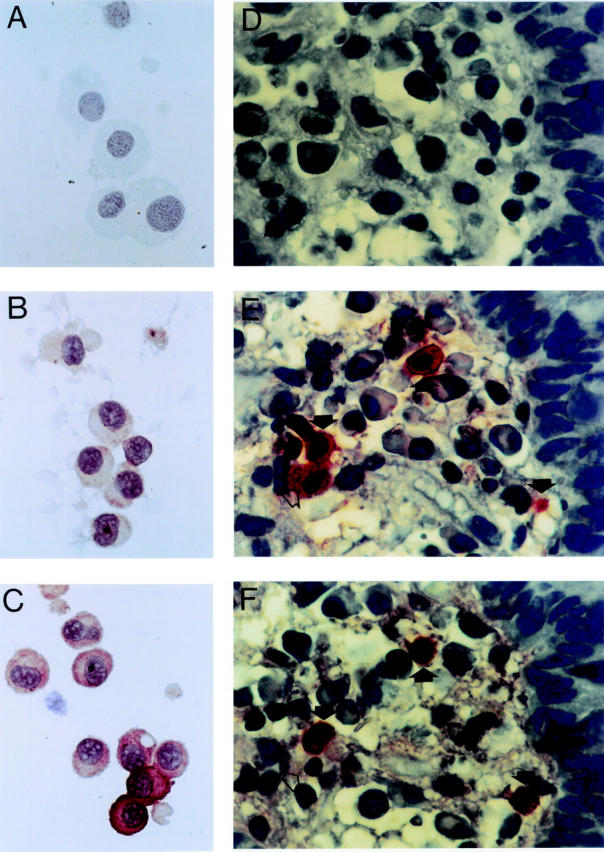
Immunostaining of human intestinal mast cells with anti-TNF-α antiserum (original magnification × 1000). (A-C) Cytocentrifuge smears of purified mast cells. A, cytocentrifuge of a mast cell preparation containing 96% mast cells, negative control (staining with a non-immune control antiserum); B, same cells as in A, unstimulated mast cells (staining with rabbit antihuman TNF-α antiserum, immunoreactive cells are stained); C, same cells as in A, stimulation of the mast cells by incubation with intestinal bacteria for six hours (staining as in B). (D-F) Immunohistochemistry of intestinal tissue sections derived from a patient with active Crohn's disease using different primary antibodies. D, negative control using non-immune control antiserum; E, staining with antihuman tryptase monoclonal antibody; F, staining with antihuman TNF-α antiserum. Closed arrows indicate TNF-α positive mast cells, open arrow indicates a TNF-α negative mast cell.
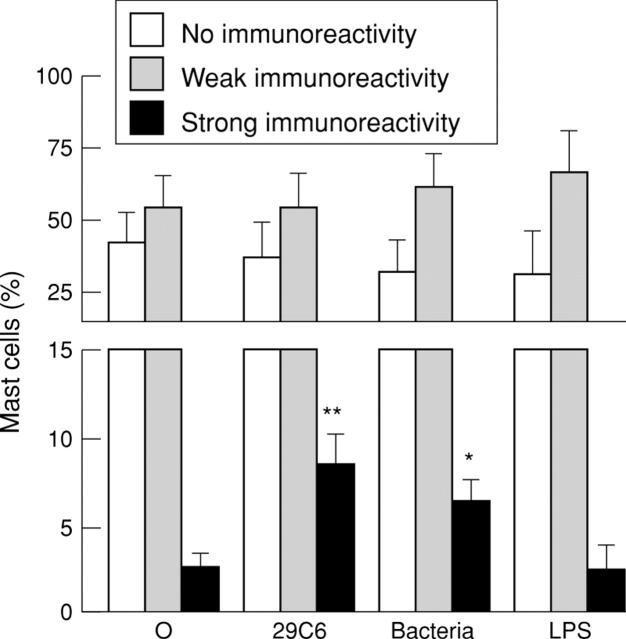
Figure 3 .
TNF-α immunoreactivity in isolated human intestinal mast cells. Cultured mast cells derived from seven cell preparations were challenged with buffer control (O), 29C6 (29C6), bacteria, or LPS. Mean (SD) percentages of mast cells with no (o), weak (+), and strong (++) immunoreactivity are shown. *p<0.05, **p<0.01 versus buffer control.
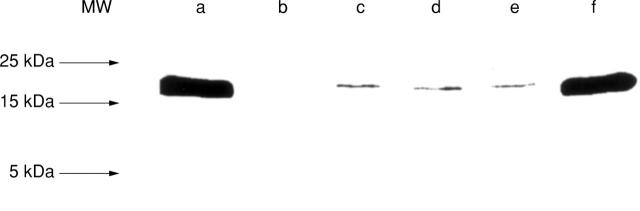
Figure 4 .
TNF-α protein in lysates of purified human intestinal mast cells. Recombinant human TNF-α (0.5 µg, lanes a,f), culture medium without cells (lane b), and lysates of 106 mast cells purified to 96% (lanes c-e). Prior to cell lysis, mast cells had been challenged for six hours with buffer control (c), 29C6 (d), or with intestinal bacteria (e). Arrows indicate the position of the molecular weight (MW) markers. The MW of the TNF-α protein bands of mast cell samples corresponds to that of recombinant TNF-α (17.9 kDa).
Figure 5 .
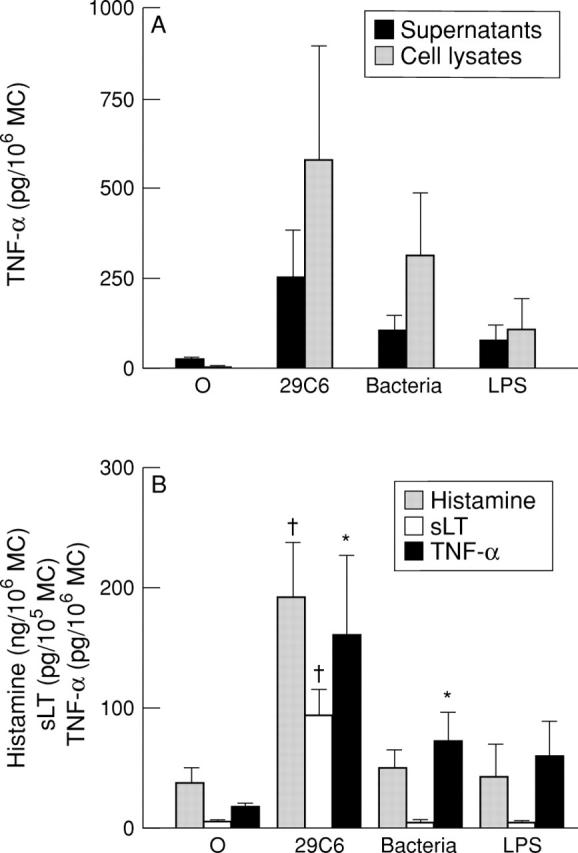
Production of TNF-α and inflammatory mediators by human intestinal mast cells. (A) Measurement of TNF-α in supernatants and cell lysates of mast cells, incubated for six hours with buffer control (O), mAb 29C6 (29C6), intestinal bacteria, or lipopolysaccharide (LPS). (B) Measurement of TNF-α, histamine, and sLT in supernatants of mast cells. *p<0.05, †p<0.005 versus buffer control.
Figure 6 .
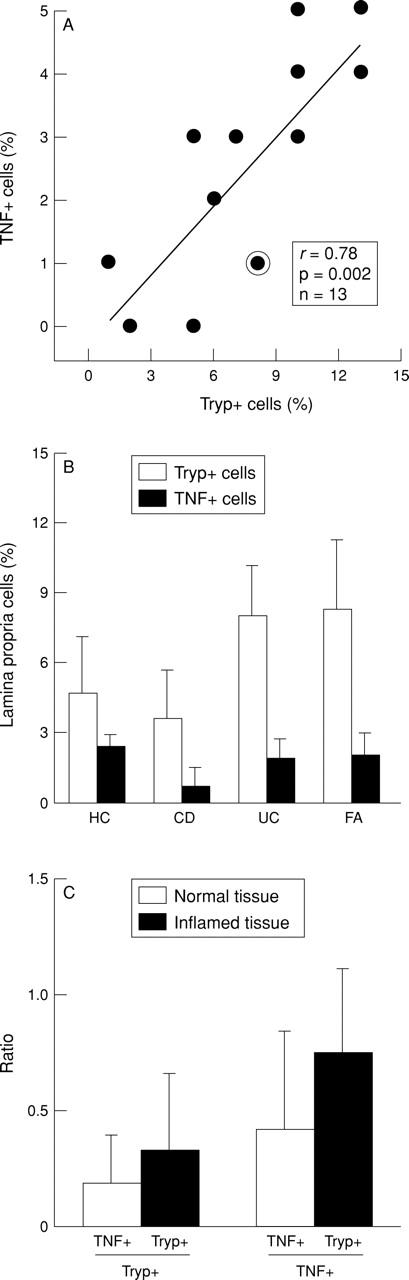
Expression of tryptase and TNF-α by human intestinal lamina propria cells. Intestinal biopsy specimens were stained with monoclonal antibody directed against tryptase (Tryp+) and TNF-α (TNF+). (A) Tryp+ and TNF+ cells were counted in two adjacent tissue sections and the percentage of Tryp+ and TNF+ lamina propria cells was calculated (n=13; the circled point represents two experiments with identical results). (B) Comparison of percentages of Tryp+ and TNF+ lamina propria cells in patients with Crohn's disease (CD, n=4), ulcerative colitis (UC, n=4), food allergy (FA, n=3), and healthy controls (HC, n= 2). (C) Ratio of TNF+ mast cells and mast cells (mast cells defined as Tryp+ cells), and ratio of TNF+ mast cells and TNF+ cells, respectively, in histologically normal (n=6) and inflamed tissue (n=7).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arock M., Zuany-Amorim C., Singer M., Benhamou M., Pretolani M. Interleukin-10 inhibits cytokine generation from mast cells. Eur J Immunol. 1996 Jan;26(1):166–170. doi: 10.1002/eji.1830260126. [DOI] [PubMed] [Google Scholar]
- Bazzoni F., Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996 Jun 27;334(26):1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- Beil W. J., Weller P. F., Peppercorn M. A., Galli S. J., Dvorak A. M. Ultrastructural immunogold localization of subcellular sites of TNF-alpha in colonic Crohn's disease. J Leukoc Biol. 1995 Sep;58(3):284–298. doi: 10.1002/jlb.58.3.284. [DOI] [PubMed] [Google Scholar]
- Benyon R. C., Bissonnette E. Y., Befus A. D. Tumor necrosis factor-alpha dependent cytotoxicity of human skin mast cells is enhanced by anti-IgE antibodies. J Immunol. 1991 Oct 1;147(7):2253–2258. [PubMed] [Google Scholar]
- Bischoff S. C., Dahinden C. A. Effect of nerve growth factor on the release of inflammatory mediators by mature human basophils. Blood. 1992 May 15;79(10):2662–2669. [PubMed] [Google Scholar]
- Bischoff S. C., Dahinden C. A. c-kit ligand: a unique potentiator of mediator release by human lung mast cells. J Exp Med. 1992 Jan 1;175(1):237–244. doi: 10.1084/jem.175.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff S. C., Schwengberg S., Raab R., Manns M. P. Functional properties of human intestinal mast cells cultured in a new culture system: enhancement of IgE receptor-dependent mediator release and response to stem cell factor. J Immunol. 1997 Dec 1;159(11):5560–5567. [PubMed] [Google Scholar]
- Bischoff S. C., Wedemeyer J., Herrmann A., Meier P. N., Trautwein C., Cetin Y., Maschek H., Stolte M., Gebel M., Manns M. P. Quantitative assessment of intestinal eosinophils and mast cells in inflammatory bowel disease. Histopathology. 1996 Jan;28(1):1–13. doi: 10.1046/j.1365-2559.1996.262309.x. [DOI] [PubMed] [Google Scholar]
- Bissonnette E. Y., Chin B., Befus A. D. Interferons differentially regulate histamine and TNF-alpha in rat intestinal mucosal mast cells. Immunology. 1995 Sep;86(1):12–17. [PMC free article] [PubMed] [Google Scholar]
- Bissonnette E. Y., Enciso J. A., Befus A. D. Inhibitory effects of sulfasalazine and its metabolites on histamine release and TNF-alpha production by mast cells. J Immunol. 1996 Jan 1;156(1):218–223. [PubMed] [Google Scholar]
- Bradding P., Mediwake R., Feather I. H., Madden J., Church M. K., Holgate S. T., Howarth P. H. TNF alpha is localized to nasal mucosal mast cells and is released in acute allergic rhinitis. Clin Exp Allergy. 1995 May;25(5):406–415. doi: 10.1111/j.1365-2222.1995.tb01071.x. [DOI] [PubMed] [Google Scholar]
- Bradding P., Okayama Y., Howarth P. H., Church M. K., Holgate S. T. Heterogeneity of human mast cells based on cytokine content. J Immunol. 1995 Jul 1;155(1):297–307. [PubMed] [Google Scholar]
- Bradding P., Roberts J. A., Britten K. M., Montefort S., Djukanovic R., Mueller R., Heusser C. H., Howarth P. H., Holgate S. T. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol. 1994 May;10(5):471–480. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- Costa J. J., Matossian K., Resnick M. B., Beil W. J., Wong D. T., Gordon J. R., Dvorak A. M., Weller P. F., Galli S. J. Human eosinophils can express the cytokines tumor necrosis factor-alpha and macrophage inflammatory protein-1 alpha. J Clin Invest. 1993 Jun;91(6):2673–2684. doi: 10.1172/JCI116506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtenacher B., Männel D. N., Hültner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996 May 2;381(6577):75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- Eklund K. K., Humphries D. E., Xia Z., Ghildyal N., Friend D. S., Gross V., Stevens R. L. Glucocorticoids inhibit the cytokine-induced proliferation of mast cells, the high affinity IgE receptor-mediated expression of TNF-alpha, and the IL-10-induced expression of chymases. J Immunol. 1997 May 1;158(9):4373–4380. [PubMed] [Google Scholar]
- Furuta G. T., Schmidt-Choudhury A., Wang M. Y., Wang Z. S., Lu L., Furlano R. I., Wershil B. K. Mast cell-dependent tumor necrosis factor alpha production participates in allergic gastric inflammation in mice. Gastroenterology. 1997 Nov;113(5):1560–1569. doi: 10.1053/gast.1997.v113.pm9352858. [DOI] [PubMed] [Google Scholar]
- Galli S. J. New concepts about the mast cell. N Engl J Med. 1993 Jan 28;328(4):257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- Gordon J. R., Galli S. J. Release of both preformed and newly synthesized tumor necrosis factor alpha (TNF-alpha)/cachectin by mouse mast cells stimulated via the Fc epsilon RI. A mechanism for the sustained action of mast cell-derived TNF-alpha during IgE-dependent biological responses. J Exp Med. 1991 Jul 1;174(1):103–107. doi: 10.1084/jem.174.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremlev S. G., Umstead T. M., Phelps D. S. Surfactant protein A regulates cytokine production in the monocytic cell line THP-1. Am J Physiol. 1997 May;272(5 Pt 1):L996–1004. doi: 10.1152/ajplung.1997.272.5.L996. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leal-Berumen I., Conlon P., Marshall J. S. IL-6 production by rat peritoneal mast cells is not necessarily preceded by histamine release and can be induced by bacterial lipopolysaccharide. J Immunol. 1994 Jun 1;152(11):5468–5476. [PubMed] [Google Scholar]
- Lerner N. B., Nocka K. H., Cole S. R., Qiu F. H., Strife A., Ashman L. K., Besmer P. Monoclonal antibody YB5.B8 identifies the human c-kit protein product. Blood. 1991 May 1;77(9):1876–1883. [PubMed] [Google Scholar]
- Lin T. J., Bissonnette E. Y., Hirsh A., Befus A. D. Stem cell factor potentiates histamine secretion by multiple mechanisms, but does not affect tumour necrosis factor-alpha release from rat mast cells. Immunology. 1996 Oct;89(2):301–307. doi: 10.1046/j.1365-2567.1996.d01-733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya R., Ikeda T., Ross E., Abraham S. N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996 May 2;381(6577):77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- Marshall J. S., Leal-Berumen I., Nielsen L., Glibetic M., Jordana M. Interleukin (IL)-10 inhibits long-term IL-6 production but not preformed mediator release from rat peritoneal mast cells. J Clin Invest. 1996 Feb 15;97(4):1122–1128. doi: 10.1172/JCI118506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson E., Van Dijk H., Van Kessel K., Verhoef J., Fleer A., Rollof J. Intracellular pathways involved in tumor necrosis factor-alpha release by human monocytes on stimulation with lipopolysaccharide or staphylococcal peptidoglycan are partly similar. J Infect Dis. 1996 Jan;173(1):212–218. doi: 10.1093/infdis/173.1.212. [DOI] [PubMed] [Google Scholar]
- Moreland L. W., Baumgartner S. W., Schiff M. H., Tindall E. A., Fleischmann R. M., Weaver A. L., Ettlinger R. E., Cohen S., Koopman W. J., Mohler K. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997 Jul 17;337(3):141–147. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- Murch S. H., Braegger C. P., Walker-Smith J. A., MacDonald T. T. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993 Dec;34(12):1705–1709. doi: 10.1136/gut.34.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama Y., Hunt T. C., Kassel O., Ashman L. K., Church M. K. Assessment of the anti-c-kit monoclonal antibody YB5.B8 in affinity magnetic enrichment of human lung mast cells. J Immunol Methods. 1994 Mar 10;169(2):153–161. doi: 10.1016/0022-1759(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Paleolog E. M., Delasalle S. A., Buurman W. A., Feldmann M. Functional activities of receptors for tumor necrosis factor-alpha on human vascular endothelial cells. Blood. 1994 Oct 15;84(8):2578–2590. [PubMed] [Google Scholar]
- Reinecker H. C., Steffen M., Witthoeft T., Pflueger I., Schreiber S., MacDermott R. P., Raedler A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993 Oct;94(1):174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riske F., Hakimi J., Mallamaci M., Griffin M., Pilson B., Tobkes N., Lin P., Danho W., Kochan J., Chizzonite R. High affinity human IgE receptor (Fc epsilon RI). Analysis of functional domains of the alpha-subunit with monoclonal antibodies. J Biol Chem. 1991 Jun 15;266(17):11245–11251. [PubMed] [Google Scholar]
- Stalder A. K., Campbell I. L. Simultaneous analysis of multiple cytokine receptor mRNAs by RNase protection assay in LPS-induced endotoxemia. Lymphokine Cytokine Res. 1994 Apr;13(2):107–112. [PubMed] [Google Scholar]
- Targan S. R., Hanauer S. B., van Deventer S. J., Mayer L., Present D. H., Braakman T., DeWoody K. L., Schaible T. F., Rutgeerts P. J. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997 Oct 9;337(15):1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- Tashiro M., Kawakami Y., Abe R., Han W., Hata D., Sugie K., Yao L., Kawakami T. Increased secretion of TNF-alpha by costimulation of mast cells via CD28 and Fc epsilon RI. J Immunol. 1997 Mar 1;158(5):2382–2389. [PubMed] [Google Scholar]
- Valent P., Besemer J., Sillaber C., Butterfield J. H., Eher R., Majdic O., Kishi K., Klepetko W., Eckersberger F., Lechner K. Failure to detect IL-3-binding sites on human mast cells. J Immunol. 1990 Nov 15;145(10):3432–3437. [PubMed] [Google Scholar]
- Van Deventer S. J. Tumour necrosis factor and Crohn's disease. Gut. 1997 Apr;40(4):443–448. doi: 10.1136/gut.40.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh L. J., Trinchieri G., Waldorf H. A., Whitaker D., Murphy G. F. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4220–4224. doi: 10.1073/pnas.88.10.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner N., Austen K. F. Heterogeneity of mast cells at multiple body sites. Fluorescent determination of avidin binding and immunofluorescent determination of chymase, tryptase, and carboxypeptidase content. Pathol Res Pract. 1993 Mar;189(2):156–162. doi: 10.1016/S0344-0338(11)80086-5. [DOI] [PubMed] [Google Scholar]
- Wershil B. K., Wang Z. S., Gordon J. R., Galli S. J. Recruitment of neutrophils during IgE-dependent cutaneous late phase reactions in the mouse is mast cell-dependent. Partial inhibition of the reaction with antiserum against tumor necrosis factor-alpha. J Clin Invest. 1991 Feb;87(2):446–453. doi: 10.1172/JCI115016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H., Anderson W. D., Cheng T., Riabowol K. T. Monitoring mRNA expression by polymerase chain reaction: the "primer-dropping" method. Anal Biochem. 1994 Dec;223(2):251–258. doi: 10.1006/abio.1994.1581. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Ramos B. F., Jakschik B. A. Neutrophil recruitment by tumor necrosis factor from mast cells in immune complex peritonitis. Science. 1992 Dec 18;258(5090):1957–1959. doi: 10.1126/science.1470922. [DOI] [PubMed] [Google Scholar]
- van Deventer S. J., Elson C. O., Fedorak R. N. Multiple doses of intravenous interleukin 10 in steroid-refractory Crohn's disease. Crohn's Disease Study Group. Gastroenterology. 1997 Aug;113(2):383–389. doi: 10.1053/gast.1997.v113.pm9247454. [DOI] [PubMed] [Google Scholar]
- van Overveld F. J., Jorens P. G., Rampart M., de Backer W., Vermeire P. A. Tumour necrosis factor stimulates human skin mast cells to release histamine and tryptase. Clin Exp Allergy. 1991 Nov;21(6):711–714. doi: 10.1111/j.1365-2222.1991.tb03200.x. [DOI] [PubMed] [Google Scholar]