Abstract
BACKGROUND—T cell responses to normal intestinal bacteria or their products may be important in the immunopathogenesis of chronic enterocolitis. AIMS—To investigate the T cell specificity and cross reactivity towards intestinal bacteria. PATIENTS/METHODS—T cell clones were isolated with phytohaemagglutinin from peripheral blood and biopsy specimens of inflamed and non-inflamed colon from five patients with inflammatory bowel disease (IBD) and two controls. T cell clones were restimulated with anaerobic Bacteroides and Bifidobacteria species, enterobacteria, and direct isolates of aerobic intestinal flora. T cell phenotype was analysed by single-cell immunocyte assay. RESULTS—Analysis of 96 T cell clones isolated from peripheral blood and biopsy specimens from two patients with IBD showed that both Bifidobacterium and Bacteroides species specifically stimulate proliferation of CD4+TCRαβ+ T cell clones from both sites and that cross reactivity exists between these anaerobic bacteria and different enterobacteria. Analysis of 210 T cell clones isolated from three patients with IBD and two controls showed that indigenous aerobic flora specifically stimulate T cell clones from peripheral blood and biopsy specimens from a foreign subject. Some of these flora specific T cell clones were cross reactive with defined enterobacteria. In addition, T cell clones stimulated by their own indigenous aerobic flora were identified in patients with IBD. CONCLUSION—Immune responses to antigens from the intestinal microflora involve a complex network of T cell specificities. Keywords: inflammatory bowel disease; T cells; intestinal flora; mucosal immunity
Full Text
The Full Text of this article is available as a PDF (171.2 KB).
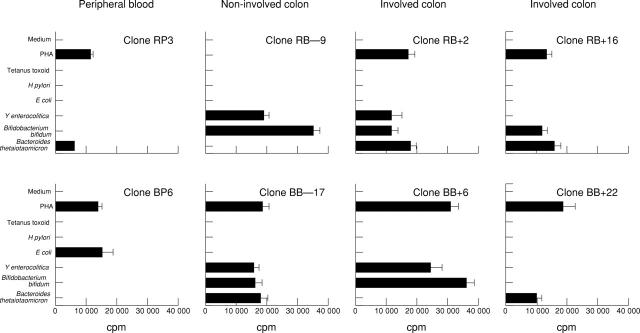
Figure 1 .
Clonal T cell responses towards Bacteroides and Bifidobacterium. T cell clones were isolated, using phytohaemagglutinin (PHA), from two patients with ulcerative colitis (patient R: peripheral blood n = 14, non-involved colon n = 12, involved colon n = 18; patient B: peripheral blood n = 12, non-involved colon n = 17, involved colon n = 23) and restimulated with the indicated antigens for 42 hours. Mean proliferation of T cell clones from peripheral blood (RP3), non-involved colon (RB−9 and BB−17), and involved colon (RB+2, RB+16, BB+6, and BB+22) stimulated by Bacteriodes thetaiotaomicron (ATCC 12290) or Bifidobacterium bifidum (ATCC 35914) is shown as measured by [3H]thymidine incorporation in triplicate cultures. Clone BP6 is representative of other T cell clones reactive with enterobacteria only. All T cell clones were CD4+TCRαβ+ by single-cell immunocyte assay.
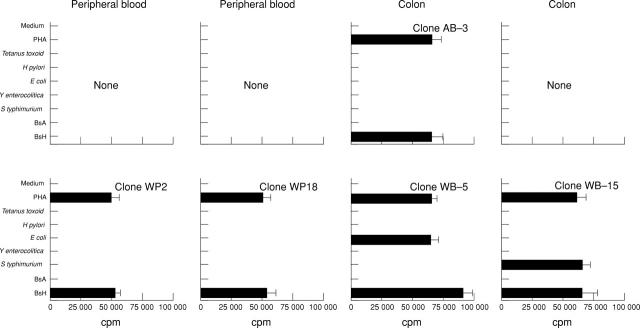
Figure 2 .
Clonal T cell responses to aerobic intestinal flora. T cell clones were isolated with phytohaemagglutinin (PHA) from two controls (patient A, bile acid induced diarrhoea: peripheral blood n = 23, colon n = 15; patient W, irritable bowel syndrome: peripheral blood n = 20, colon n = 19) and restimulated with the indicated antigens for 42 hours. Aerobic intestinal flora was obtained from intestinal biopsy specimens touched to blood agar plates and grown under aerobic conditions. Sonicates from outgrowing bacteria were used as BsA (sonicates and mononuclear cells from the same person) or BsH (sonicates and mononuclear cells from different people). Mean proliferation of T cell clones from peripheral blood (WP2 and WP18) and colon (AB−3, WB−5, and WB−15) stimulated by BsA or BsH is shown as measured by [3H]thymidine incorporation of triplicate cultures. All T cell clones were CD4+TCRαβ+ by single-cell immunocyte assay. Incubation of antigen presenting cells with the indicated stimuli without T cell clones resulted in <500 cpm.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auer I. O., Röder A., Wensinck F., van de Merwe J. P., Schmidt H. Selected bacterial antibodies in Crohn's disease and ulcerative colitis. Scand J Gastroenterol. 1983 Mar;18(2):217–223. doi: 10.3109/00365528309181586. [DOI] [PubMed] [Google Scholar]
- Bernet M. F., Brassart D., Neeser J. R., Servin A. L. Adhesion of human bifidobacterial strains to cultured human intestinal epithelial cells and inhibition of enteropathogen-cell interactions. Appl Environ Microbiol. 1993 Dec;59(12):4121–4128. doi: 10.1128/aem.59.12.4121-4128.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I., Hayllar J., Smethurst P., Price A., Gumpel M. J. Metronidazole reduces intestinal inflammation and blood loss in non-steroidal anti-inflammatory drug induced enteropathy. Gut. 1992 Sep;33(9):1204–1208. doi: 10.1136/gut.33.9.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dombal F. T., Burton I. L., Clamp S. E., Goligher J. C. Short-term course and prognosis of Crohn's disease. Gut. 1974 Jun;15(6):435–443. doi: 10.1136/gut.15.6.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Tommaso A., Xiang Z., Bugnoli M., Pileri P., Figura N., Bayeli P. F., Rappuoli R., Abrignani S., De Magistris M. T. Helicobacter pylori-specific CD4+ T-cell clones from peripheral blood and gastric biopsies. Infect Immun. 1995 Mar;63(3):1102–1106. doi: 10.1128/iai.63.3.1102-1106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchmann R., Kaiser I., Hermann E., Mayet W., Ewe K., Meyer zum Büschenfelde K. H. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995 Dec;102(3):448–455. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchmann R., Schmitt E., Knolle P., Meyer zum Büschenfelde K. H., Neurath M. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur J Immunol. 1996 Apr;26(4):934–938. doi: 10.1002/eji.1830260432. [DOI] [PubMed] [Google Scholar]
- Duchmann R., Strober W., James S. P. Quantitative measurement of human T-cell receptor V beta subfamilies by reverse transcription-polymerase chain reaction using synthetic internal mRNA standards. DNA Cell Biol. 1993 Apr;12(3):217–225. doi: 10.1089/dna.1993.12.217. [DOI] [PubMed] [Google Scholar]
- Foo M. C., Lee A. Antigenic cross-reaction between mouse intestine and a member of the autochthonous microflora. Infect Immun. 1974 Jun;9(6):1066–1069. doi: 10.1128/iai.9.6.1066-1069.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss I. J., Neurath M., Boirivant M., Klein J. S., de la Motte C., Strong S. A., Fiocchi C., Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996 Aug 1;157(3):1261–1270. [PubMed] [Google Scholar]
- Gardiner K. R., Anderson N. H., Rowlands B. J., Barbul A. Colitis and colonic mucosal barrier dysfunction. Gut. 1995 Oct;37(4):530–535. doi: 10.1136/gut.37.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner K. R., Erwin P. J., Anderson N. H., Barr J. G., Halliday M. I., Rowlands B. J. Colonic bacteria and bacterial translocation in experimental colitis. Br J Surg. 1993 Apr;80(4):512–516. doi: 10.1002/bjs.1800800436. [DOI] [PubMed] [Google Scholar]
- Giaffer M. H., Holdsworth C. D., Duerden B. I. Virulence properties of Escherichia coli strains isolated from patients with inflammatory bowel disease. Gut. 1992 May;33(5):646–650. doi: 10.1136/gut.33.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. R., Wang X. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J Appl Bacteriol. 1994 Oct;77(4):412–420. doi: 10.1111/j.1365-2672.1994.tb03443.x. [DOI] [PubMed] [Google Scholar]
- Hammer R. E., Maika S. D., Richardson J. A., Tang J. P., Taurog J. D. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: an animal model of HLA-B27-associated human disorders. Cell. 1990 Nov 30;63(5):1099–1112. doi: 10.1016/0092-8674(90)90512-d. [DOI] [PubMed] [Google Scholar]
- Hermann E., Fleischer B., Mayet W. J., Poralla T., Meyer zum Büschenfelde K. H. Response of synovial fluid T cell clones to Yersinia enterocolitica antigens in patients with reactive Yersinia arthritis. Clin Exp Immunol. 1989 Mar;75(3):365–370. [PMC free article] [PubMed] [Google Scholar]
- Keighley M. R., Arabi Y., Dimock F., Burdon D. W., Allan R. N., Alexander-Williams J. Influence of inflammatory bowel disease on intestinal microflora. Gut. 1978 Dec;19(12):1099–1104. doi: 10.1136/gut.19.12.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten C. M., McCluskey R. T., Boyle L. A., Kurnick J. T. Escherichia coli and Pseudomonas aeruginosa induce expansion of V delta 2 cells in adult peripheral blood, but of V delta 1 cells in cord blood. J Immunol. 1996 Aug 15;157(4):1613–1619. [PubMed] [Google Scholar]
- Kersten C. M., McCluskey R. T., Shaw Warren H., Kurnick J. T. Responses of human T cells to dominant discrete protein antigens of Escherichia coli and Pseudomonas aeruginosa. Scand J Immunol. 1994 Aug;40(2):151–157. doi: 10.1111/j.1365-3083.1994.tb03444.x. [DOI] [PubMed] [Google Scholar]
- Krook A., Lindström B., Kjellander J., Järnerot G., Bodin L. Relation between concentrations of metronidazole and Bacteroides spp in faeces of patients with Crohn's disease and healthy individuals. J Clin Pathol. 1981 Jun;34(6):645–650. doi: 10.1136/jcp.34.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Mee A. S., McLaughlin J. E., Hodgson H. J., Jewell D. P. Chronic immune colitis in rabbits. Gut. 1979 Jan;20(1):1–5. doi: 10.1136/gut.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath M. F., Fuss I., Kelsall B. L., Presky D. H., Waegell W., Strober W. Experimental granulomatous colitis in mice is abrogated by induction of TGF-beta-mediated oral tolerance. J Exp Med. 1996 Jun 1;183(6):2605–2616. doi: 10.1084/jem.183.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath M. F., Fuss I., Kelsall B. L., Stüber E., Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995 Nov 1;182(5):1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk A. B., Franklin M. L., Cisneros R. L. Production of experimental ulcerative colitis in gnotobiotic guinea pigs with simplified microflora. Infect Immun. 1981 Apr;32(1):225–231. doi: 10.1128/iai.32.1.225-231.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peach S. L., Drasar B. S., Hawley P. R., Hill M. J., Marks C. G. Proceedings: Mucosal flora of the human colon. Gut. 1975 Oct;16(10):824–824. [PubMed] [Google Scholar]
- Peppercorn M. A. Is there a role for antibiotics as primary therapy in Crohn's ileitis? J Clin Gastroenterol. 1993 Oct;17(3):235–237. doi: 10.1097/00004836-199310000-00013. [DOI] [PubMed] [Google Scholar]
- Pirzer U., Schönhaar A., Fleischer B., Hermann E., Meyer zum Büschenfelde K. H. Reactivity of infiltrating T lymphocytes with microbial antigens in Crohn's disease. Lancet. 1991 Nov 16;338(8777):1238–1239. doi: 10.1016/0140-6736(91)92104-a. [DOI] [PubMed] [Google Scholar]
- Probert C. S., Chott A., Turner J. R., Saubermann L. J., Stevens A. C., Bodinaku K., Elson C. O., Balk S. P., Blumberg R. S. Persistent clonal expansions of peripheral blood CD4+ lymphocytes in chronic inflammatory bowel disease. J Immunol. 1996 Oct 1;157(7):3183–3191. [PubMed] [Google Scholar]
- Rath H. C., Herfarth H. H., Ikeda J. S., Grenther W. B., Hamm T. E., Jr, Balish E., Taurog J. D., Hammer R. E., Wilson K. H., Sartor R. B. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996 Aug 15;98(4):945–953. doi: 10.1172/JCI118878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A., Asano T. Resistance of germfree rats to indomethacin-induced intestinal lesions. Prostaglandins. 1977 Aug;14(2):333–341. doi: 10.1016/0090-6980(77)90178-2. [DOI] [PubMed] [Google Scholar]
- Rodloff A. C., Widera P., Ehlers S., Montag T., Lucas M., Schmidt G., Hahn H. Suppression of blastogenic transformation of lymphocytes by Bacteroides fragilis in vitro and in vivo. Zentralbl Bakteriol. 1990 Dec;274(3):406–416. doi: 10.1016/s0934-8840(11)80699-7. [DOI] [PubMed] [Google Scholar]
- Ruseler-van Embden J. G., Both-Patoir H. C. Anaerobic gram-negative faecal flora in patients with Crohn's disease and healthy subjects. Antonie Van Leeuwenhoek. 1983 Jun;49(2):125–132. doi: 10.1007/BF00393670. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P., Goboes K., Peeters M., Hiele M., Penninckx F., Aerts R., Kerremans R., Vantrappen G. Effect of faecal stream diversion on recurrence of Crohn's disease in the neoterminal ileum. Lancet. 1991 Sep 28;338(8770):771–774. doi: 10.1016/0140-6736(91)90663-a. [DOI] [PubMed] [Google Scholar]
- Saavedra J. M., Bauman N. A., Oung I., Perman J. A., Yolken R. H. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet. 1994 Oct 15;344(8929):1046–1049. doi: 10.1016/s0140-6736(94)91708-6. [DOI] [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schorle H., Schimpl A., Feller A. C., Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993 Oct 22;75(2):253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Sartor R. B., Cromartie W. J., Powell D. W., Schwab J. H. Granulomatous enterocolitis induced in rats by purified bacterial cell wall fragments. Gastroenterology. 1985 Sep;89(3):587–595. doi: 10.1016/0016-5085(85)90455-x. [DOI] [PubMed] [Google Scholar]
- Schreiber S., MacDermott R. P., Raedler A., Pinnau R., Bertovich M. J., Nash G. S. Increased activation of isolated intestinal lamina propria mononuclear cells in inflammatory bowel disease. Gastroenterology. 1991 Oct;101(4):1020–1030. doi: 10.1016/0016-5085(91)90729-5. [DOI] [PubMed] [Google Scholar]
- Simon G. L., Gorbach S. L. Intestinal flora in health and disease. Gastroenterology. 1984 Jan;86(1):174–193. [PubMed] [Google Scholar]
- Sutherland L., Singleton J., Sessions J., Hanauer S., Krawitt E., Rankin G., Summers R., Mekhjian H., Greenberger N., Kelly M. Double blind, placebo controlled trial of metronidazole in Crohn's disease. Gut. 1991 Sep;32(9):1071–1075. doi: 10.1136/gut.32.9.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Merwe J. P., Schröder A. M., Wensinck F., Hazenberg M. P. The obligate anaerobic faecal flora of patients with Crohn's disease and their first-degree relatives. Scand J Gastroenterol. 1988 Nov;23(9):1125–1131. doi: 10.3109/00365528809090179. [DOI] [PubMed] [Google Scholar]
- Van de Merwe J. P., Stegeman J. H., Hazenberg M. P. The resident faecal flora is determined by genetic characteristics of the host. Implications for Crohn's disease? Antonie Van Leeuwenhoek. 1983 Jun;49(2):119–124. doi: 10.1007/BF00393669. [DOI] [PubMed] [Google Scholar]
- Videla S., Vilaseca J., Guarner F., Salas A., Treserra F., Crespo E., Antolín M., Malagelada J. R. Role of intestinal microflora in chronic inflammation and ulceration of the rat colon. Gut. 1994 Aug;35(8):1090–1097. doi: 10.1136/gut.35.8.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells C. L., van de Westerlo E. M., Jechorek R. P., Feltis B. A., Wilkins T. D., Erlandsen S. L. Bacteroides fragilis enterotoxin modulates epithelial permeability and bacterial internalization by HT-29 enterocytes. Gastroenterology. 1996 May;110(5):1429–1437. doi: 10.1053/gast.1996.v110.pm8613048. [DOI] [PubMed] [Google Scholar]
- Wyatt J., Vogelsang H., Hübl W., Waldhöer T., Lochs H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet. 1993 Jun 5;341(8858):1437–1439. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]