Abstract
BACKGROUND/AIMS—Replication deficient recombinant adenoviruses represent an efficient means of transferring genes in vivo into a wide variety of dividing and quiescent cells from many different organs. Although the gastrointestinal tract is a potentially attractive target for gene therapy approaches, only a few studies on the use of viral gene transfer vehicles in the gut have been reported. The prospects of using recombinant adenoviruses for gene delivery into epithelial and subepithelial cells of the normal and inflamed colon are here analysed. METHODS—An E1/E3 deleted recombinant adenovirus (denoted AdCMVβGal) and an adenovirus with modified fibre structure (denoted AdZ.F(pk7)) both expressing the bacterial lacZ gene under the control of a human cytomegalovirus promoter were used for reporter gene expression in vitro and in vivo. β-Galactosidase activity was determined by specific chemiluminescent reporter gene assay. RESULTS—Intravenous or intraperitoneal injection of AdCMVβGal into healthy Balb/c mice caused strong reporter gene expression in the liver and spleen but not in the colon. In contrast, local administration of AdCMVβGal resulted in high reporter gene expression in colonic epithelial cells and lamina propria mononuclear cells. A local route of adenovirus administration in mice with experimental colitis induced by the hapten reagent trinitrobenzenesulphonic acid was next evaluated. Interestingly, rectal administration of AdCMVβGal caused a higher β-galactosidase activity in isolated lamina propria cells from infected mice with experimental colitis than in those from controls. Furthermore, isolated lamina propria cells from mice with colitis infected in vitro showed a significant increase in reporter gene activity compared with controls. Finally, AdZ.F(pk7) adenoviruses with modified fibre structure produced 10- to 40-fold higher reporter gene activity in spleen T cells and lamina propria mononuclear cells of colitic mice compared with standard AdCMVβGal vectors. CONCLUSIONS—Local administration of recombinant adenoviruses with normal or modified fibre structure could provide a new reliable method for targeted gene expression in the inflamed colon. Such gene delivery could be used to specifically express signal transduction proteins with therapeutic potential in inflamed colonic tissue. In particular, adenoviruses with modified fibre structure may be useful in T cell directed therapies in intestinal inflammation. Keywords: adenovirus; gene transfer; colitis; colon
Full Text
The Full Text of this article is available as a PDF (229.5 KB).
Figure 1 .
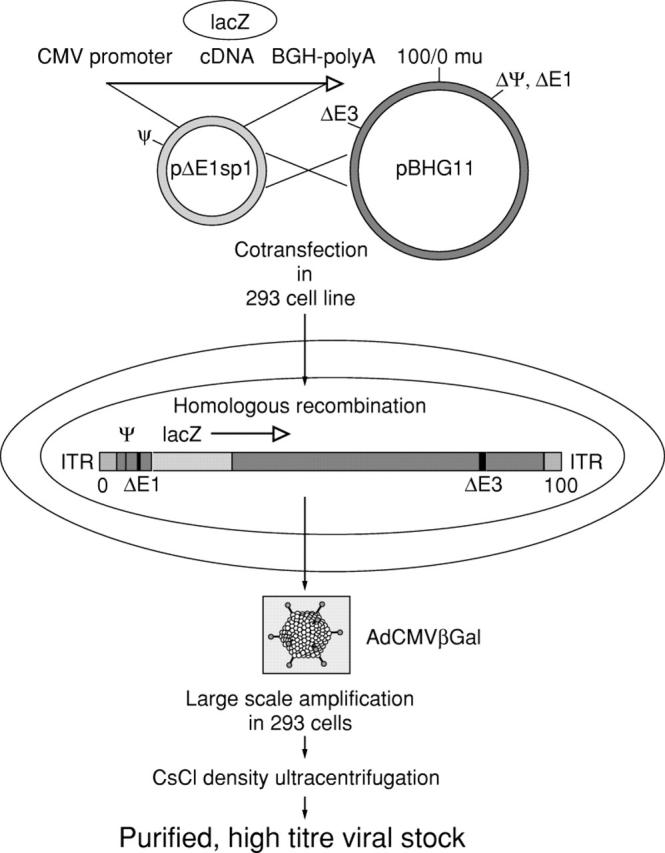
Strategy for generation and propagation of Ad5 vectors. This vector can be used to obtain expression vectors for cytokine signalling proteins (S Wirtz, P R Galle & M F Neurath, unpublished work) or reporter genes such as β-galactosidase. To obtain the AdCMVβGal vector, the entire β-galactosidase coding region was inserted into the Ad5 pBHG11 vector via homologous recombination using a shuttle vector (pΔE1sp1) and cotransfection strategies in 293 cells. After recombination, large scale amplification of AdCMVβGal was performed in 293 cells. Finally, high titre viral stocks were generated and used for in vitro and in vivo studies. CMV, cytomegalovirus; ITR, inverted terminal repeat.
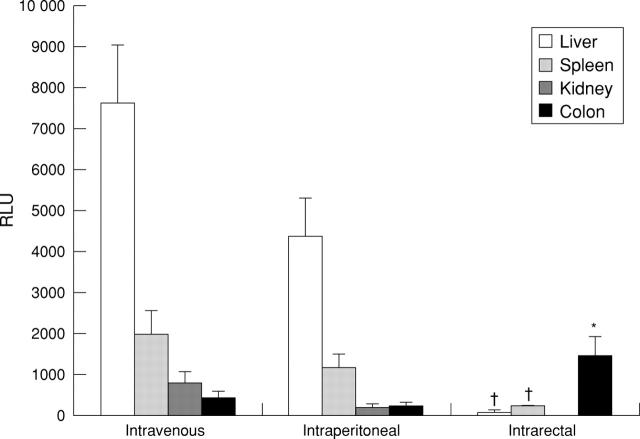
Figure 2 .
β-Galactosidase activities after different routes of reporter vector administration. Six to eight week old healthy Balb/c mice were infected with 1 × 109 pfu AdCMVβGal by the indicated administration routes. After three days, organs were removed and homogenised, and luminescent β-galactosidase activity was determined. Results are expressed as mean (SD) from three independent experiments (three mice/group) and are reported as increase in enzyme activity (relative light units (RLU))/100 mg of tissue) compared with untreated control mice. * and † indicate significantly (p<0.05) increased or decreased respectively RLU values compared with the other two administration routes. nd, not determined.
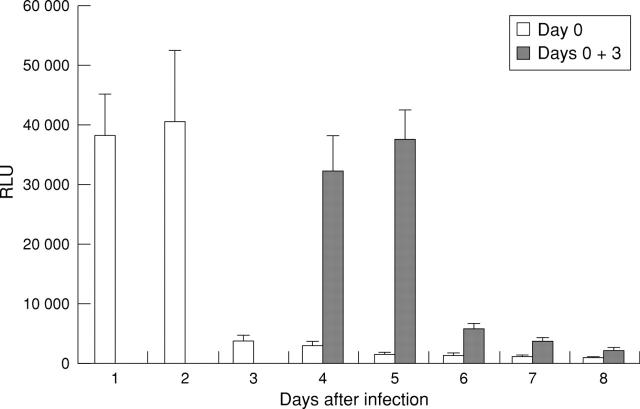
Figure 3 .
Time course of β-galactosidase expression in the colon of Balb/c mice. Six to eight week old Balb/c mice were infected at days 0 and 3 with 1 × 109 pfu AdCMVβGal via the rectum. The colon was removed at the indicated time points, and luminescent β-galactosidase activity was determined in colonic homogenates. Results are expressed as mean (SD) from three independent experiments (three mice/group) and represent an increase in enzyme activity (relative light units (RLU)) compared with uninfected control mice.
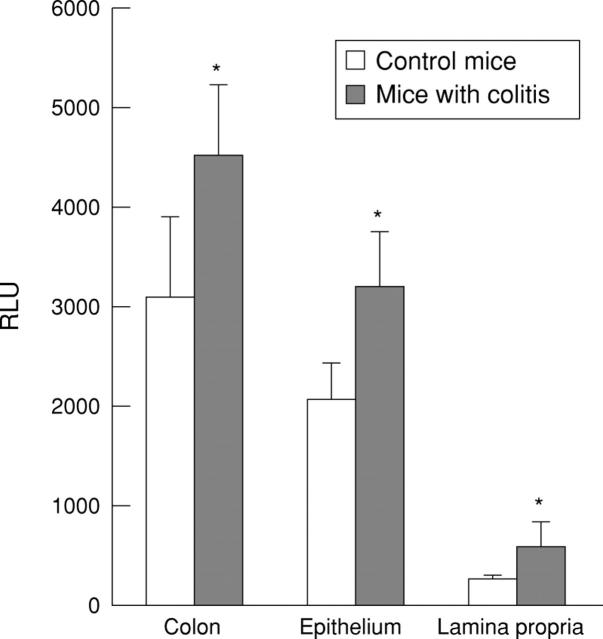
Figure 4 .
β-Galactosidase activities in the colon of mice with experimental colitis. Six to eight week old Balb/c mice with or without trinitrobenzenesulphonic acid (TNBS) induced colitis were infected with 1 × 109 pfu AdCMVβGal via the rectum. After three days the colon was removed and homogenised. In addition, epithelial and lamina propria cells were isolated and luminescent β-galactosidase activity was determined. Results are expressed as mean (SD) from three independent experiments (three mice/group) and represent an increase in enzyme activity (relative light units (RLU)) compared with untreated control mice. Asterisks indicate significantly (p<0.05) increased RLU values compared with the control group.
Figure 5 .
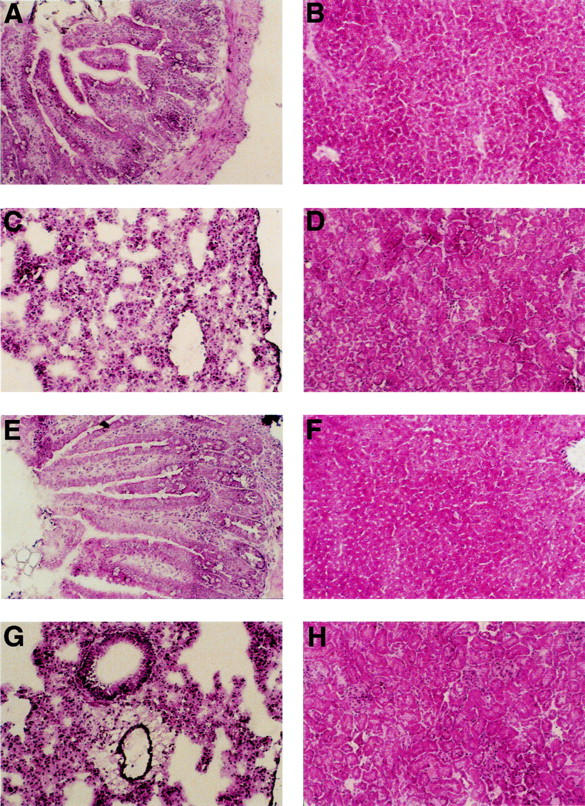
Histological analysis of the small bowel (A, E), liver (B, F), lung (C, G), and kidney (D, H) of mice with experimental colitis given AdCMVβGal or phosphate buffered saline (PBS). Eight week old Balb/c mice with trinitrobenzenesulphonic acid (TNBS) induced colitis were infected with 1 × 109 pfu AdCMVβGal via the rectum. After seven days, organs from AdCMVβGal treated (lower panel) and PBS treated control (upper panel) mice were removed. Cryosections were made and stained with haematoxylin/eosin. No inflammatory reaction was noted upon adenoviral gene delivery. Additional experiments after a second administration of AdCMVβGal after 14 days showed similar results (not shown). Magnification × 200.
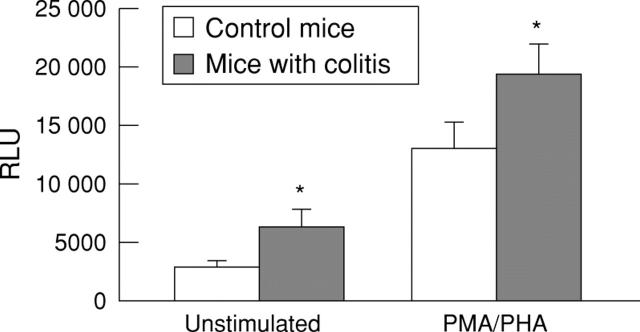
Figure 6 .
β-Galactosidase activities in infected lamina propria cells (LPMCs). LPMCs from healthy Balb/c mice or mice with trinitrobenzenesulphonic acid (TNBS) induced colitis were isolated. Then 1 × 106 unstimulated or phorbol ester/phytohaemagglutinin (PMA/PHA) stimulated cells were infected with AdCMVβGal at a multiplicity of infection of 1000. After three days the cells were lysed and luminescent β-galactosidase activity was determined. Results are expressed as mean (SD) from three independent experiments (three mice/group) and represent an increase in enzyme activity (relative light units (RLU)) compared with untreated control mice. Asterisks indicate significantly (p<0.05) increased RLU values compared with the control group.
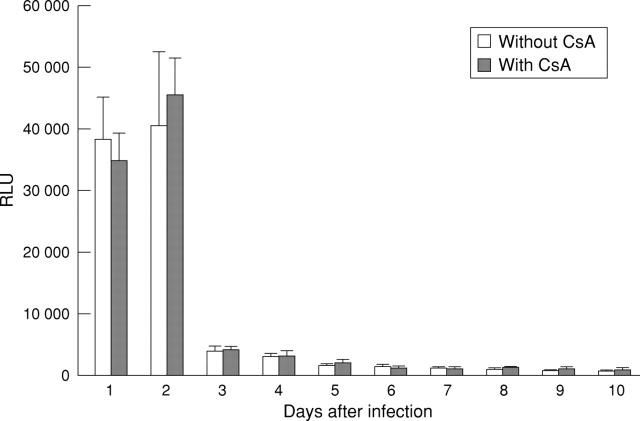
Figure 7 .
Time course of reporter gene expression in colitic mice with or without daily treatment with cyclosporin A (CsA). Mice with trinitrobenzenesulphonic acid (TNBS) induced colitis were infected with 1 × 109 pfu AdCMVβGal and treated with CsA as indicated. Mice were killed at the indicated time points, and luminescent β-galactosidase activity in homogenised colonic specimens was determined as specified in Methods. Results represent mean (SD) from three independent experiments (three mice/group) and represent an increase in enzyme activity (relative light units (RLU))/100 mg tissue compared with untreated control mice.
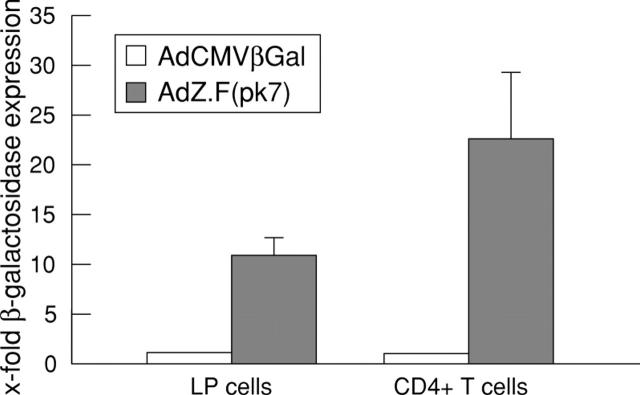
Figure 8 .
Adenoviral gene delivery to colonic lamina propria mononuclear cells (LPMCs) and spleen CD4 T cells using viruses with normal or modified fibre structure. β-Galactosidase activities in AdCMVβGal or AdZ.F(pk7) infected spleen CD4 T cells from healthy control mice and LPMCs from mice with trinitrobenzenesulphonic acid (TNBS) induced colitis were measured. A total of 1 × 106 cells were infected with AdCMVβGal at a multiplicity of infection of 1000. After three days the cells were lysed, and luminescent β-galactosidase activity was determined. Results represent mean (SD) from three independent experiments.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton E. W., Middleton P. G., Caplen N. J., Smith S. N., Steel D. M., Munkonge F. M., Jeffery P. K., Geddes D. M., Hart S. L., Williamson R. Non-invasive liposome-mediated gene delivery can correct the ion transport defect in cystic fibrosis mutant mice. Nat Genet. 1993 Oct;5(2):135–142. doi: 10.1038/ng1093-135. [DOI] [PubMed] [Google Scholar]
- Bett A. J., Haddara W., Prevec L., Graham F. L. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8802–8806. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. R., Thiele D. L., Silva M., Beutler B. Adenoviral vectors given intravenously to immunocompromised mice yield stable transduction of the colonic epithelium. Gastroenterology. 1997 May;112(5):1586–1594. doi: 10.1016/s0016-5085(97)70040-4. [DOI] [PubMed] [Google Scholar]
- Cheng D. Y., Kolls J. K., Lei D., Noel R. A. In vivo and in vitro gene transfer and expression in rat intestinal epithelial cells by E1-deleted adenoviral vector. Hum Gene Ther. 1997 Apr 10;8(6):755–764. doi: 10.1089/hum.1997.8.6-755. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Hirschowitz E., Lieberman M., Daly J., Kazam E., Henschke C., Yankelevitz D., Kemeny N., Silverstein R., Ohwada A. Phase I study of direct administration of a replication deficient adenovirus vector containing the E. coli cytosine deaminase gene to metastatic colon carcinoma of the liver in association with the oral administration of the pro-drug 5-fluorocytosine. Hum Gene Ther. 1997 May 20;8(8):985–1001. doi: 10.1089/hum.1997.8.8-985. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., McElvaney N. G., Rosenfeld M. A., Chu C. S., Mastrangeli A., Hay J. G., Brody S. L., Jaffe H. A., Eissa N. T., Danel C. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat Genet. 1994 Sep;8(1):42–51. doi: 10.1038/ng0994-42. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Sartor R. B., Tennyson G. S., Riddell R. H. Experimental models of inflammatory bowel disease. Gastroenterology. 1995 Oct;109(4):1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- Gao G. P., Yang Y., Wilson J. M. Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy. J Virol. 1996 Dec;70(12):8934–8943. doi: 10.1128/jvi.70.12.8934-8943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Hardy S., Kitamura M., Harris-Stansil T., Dai Y., Phipps M. L. Construction of adenovirus vectors through Cre-lox recombination. J Virol. 1997 Mar;71(3):1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargest R., Williamson R. Prophylactic gene therapy for cancer. Gene Ther. 1996 Feb;3(2):97–102. [PubMed] [Google Scholar]
- Hogaboam C. M., Vallance B. A., Kumar A., Addison C. L., Graham F. L., Gauldie J., Collins S. M. Therapeutic effects of interleukin-4 gene transfer in experimental inflammatory bowel disease. J Clin Invest. 1997 Dec 1;100(11):2766–2776. doi: 10.1172/JCI119823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Endo R. I., Nemerow G. R. Upregulation of integrins alpha v beta 3 and alpha v beta 5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J Virol. 1995 Apr;69(4):2257–2263. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard J., Lochmüller H., Acsadi G., Jani A., Holland P., Guérin C., Massie B., Karpati G. Differential short-term transduction efficiency of adult versus newborn mouse tissues by adenoviral recombinants. Exp Mol Pathol. 1995 Apr;62(2):131–143. doi: 10.1006/exmp.1995.1015. [DOI] [PubMed] [Google Scholar]
- Huard J., Lochmüller H., Acsadi G., Jani A., Massie B., Karpati G. The route of administration is a major determinant of the transduction efficiency of rat tissues by adenoviral recombinants. Gene Ther. 1995 Mar;2(2):107–115. [PubMed] [Google Scholar]
- Jobin C., Panja A., Hellerbrand C., Iimuro Y., Didonato J., Brenner D. A., Sartor R. B. Inhibition of proinflammatory molecule production by adenovirus-mediated expression of a nuclear factor kappaB super-repressor in human intestinal epithelial cells. J Immunol. 1998 Jan 1;160(1):410–418. [PubMed] [Google Scholar]
- Kass-Eisler A., Falck-Pedersen E., Alvira M., Rivera J., Buttrick P. M., Wittenberg B. A., Cipriani L., Leinwand L. A. Quantitative determination of adenovirus-mediated gene delivery to rat cardiac myocytes in vitro and in vivo. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11498–11502. doi: 10.1073/pnas.90.24.11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Le Gal La Salle G., Robert J. J., Berrard S., Ridoux V., Stratford-Perricaudet L. D., Perricaudet M., Mallet J. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993 Feb 12;259(5097):988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- Li M., Lonial H., Citarella R., Lindh D., Colina L., Kramer R. Tumor inhibitory activity of anti-ras ribozymes delivered by retroviral gene transfer. Cancer Gene Ther. 1996 Jul-Aug;3(4):221–229. [PubMed] [Google Scholar]
- Li Q., Kay M. A., Finegold M., Stratford-Perricaudet L. D., Woo S. L. Assessment of recombinant adenoviral vectors for hepatic gene therapy. Hum Gene Ther. 1993 Aug;4(4):403–409. doi: 10.1089/hum.1993.4.4-403. [DOI] [PubMed] [Google Scholar]
- Lozier J. N., Yankaskas J. R., Ramsey W. J., Chen L., Berschneider H., Morgan R. A. Gut epithelial cells as targets for gene therapy of hemophilia. Hum Gene Ther. 1997 Aug 10;8(12):1481–1490. doi: 10.1089/hum.1997.8.12-1481. [DOI] [PubMed] [Google Scholar]
- Macdonald T. T. Viral vectors expressing immunoregulatory cytokines to treat inflammatory bowel disease. Gut. 1998 Apr;42(4):460–461. doi: 10.1136/gut.42.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath M. F., Fuss I., Kelsall B. L., Presky D. H., Waegell W., Strober W. Experimental granulomatous colitis in mice is abrogated by induction of TGF-beta-mediated oral tolerance. J Exp Med. 1996 Jun 1;183(6):2605–2616. doi: 10.1084/jem.183.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath M. F., Fuss I., Kelsall B. L., Stüber E., Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995 Nov 1;182(5):1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath M. F., Pettersson S., Meyer zum Büschenfelde K. H., Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996 Sep;2(9):998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- Noel R. A., Shukla P., Henning S. J. Optimization of gene transfer into intestinal epithelial cells using a retroviral vector. J Pediatr Gastroenterol Nutr. 1994 Jul;19(1):43–49. doi: 10.1097/00005176-199407000-00007. [DOI] [PubMed] [Google Scholar]
- Ohno T., Gordon D., San H., Pompili V. J., Imperiale M. J., Nabel G. J., Nabel E. G. Gene therapy for vascular smooth muscle cell proliferation after arterial injury. Science. 1994 Aug 5;265(5173):781–784. doi: 10.1126/science.8047883. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K. Inflammatory bowel disease (1) N Engl J Med. 1991 Sep 26;325(13):928–937. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- Powrie F. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 1995 Aug;3(2):171–174. doi: 10.1016/1074-7613(95)90086-1. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Siegfried W., Yoshimura K., Yoneyama K., Fukayama M., Stier L. E., Päkkö P. K., Gilardi P., Stratford-Perricaudet L. D., Perricaudet M. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991 Apr 19;252(5004):431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schorle H., Schimpl A., Feller A. C., Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993 Oct 22;75(2):253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Schmid R. M., Weidenbach H., Draenert G. F., Lerch M. M., Liptay S., Schorr J., Beckh K. H., Adler G. Liposome mediated in vivo gene transfer into different tissues of the gastrointestinal tract. Z Gastroenterol. 1994 Dec;32(12):665–670. [PubMed] [Google Scholar]
- Stewart A. K., Lassam N. J., Graham F. L., Gauldie J., Addison C. L., Bailey D. J., Dessureault S., Dubé I. D., Gallenger S., Krajden M. A phase I study of adenovirus mediated gene transfer of interleukin 2 cDNA into metastatic breast cancer or melanoma. Hum Gene Ther. 1997 Jul 20;8(11):1403–1414. doi: 10.1089/hum.1997.8.11-1403. [DOI] [PubMed] [Google Scholar]
- Strober W., Kelsall B., Fuss I., Marth T., Ludviksson B., Ehrhardt R., Neurath M. Reciprocal IFN-gamma and TGF-beta responses regulate the occurrence of mucosal inflammation. Immunol Today. 1997 Feb;18(2):61–64. doi: 10.1016/s0167-5699(97)01000-1. [DOI] [PubMed] [Google Scholar]
- Targan S. R., Hanauer S. B., van Deventer S. J., Mayer L., Present D. H., Braakman T., DeWoody K. L., Schaible T. F., Rutgeerts P. J. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997 Oct 9;337(15):1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- Trapnell B. C., Gorziglia M. Gene therapy using adenoviral vectors. Curr Opin Biotechnol. 1994 Dec;5(6):617–625. doi: 10.1016/0958-1669(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Ueno Y., Yajima T., Okamoto S., Hayashi T., Yamazaki M., Iwao Y., Ishii H., Habu S., Uehira M. Interleukin 7 transgenic mice develop chronic colitis with decreased interleukin 7 protein accumulation in the colonic mucosa. J Exp Med. 1998 Feb 2;187(3):389–402. doi: 10.1084/jem.187.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook C. A., Chmura S. J., Arenas R. B., Kim S. Y., Otto G. Human APC gene expression in rodent colonic epithelium in vivo using liposomal gene delivery. Hum Mol Genet. 1994 Nov;3(11):2005–2010. doi: 10.1093/hmg/3.11.2005. [DOI] [PubMed] [Google Scholar]
- Wickham T. J., Lee G. M., Titus J. A., Sconocchia G., Bakács T., Kovesdi I., Segal D. M. Targeted adenovirus-mediated gene delivery to T cells via CD3. J Virol. 1997 Oct;71(10):7663–7669. doi: 10.1128/jvi.71.10.7663-7669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham T. J., Mathias P., Cheresh D. A., Nemerow G. R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993 Apr 23;73(2):309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Wilson J. M. Adenoviruses as gene-delivery vehicles. N Engl J Med. 1996 May 2;334(18):1185–1187. doi: 10.1056/NEJM199605023341809. [DOI] [PubMed] [Google Scholar]
- Yang Y., Nunes F. A., Berencsi K., Furth E. E., Gönczöl E., Wilson J. M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Wilson J. M. Clearance of adenovirus-infected hepatocytes by MHC class I-restricted CD4+ CTLs in vivo. J Immunol. 1995 Sep 1;155(5):2564–2570. [PubMed] [Google Scholar]
- van Deventer S. J., Elson C. O., Fedorak R. N. Multiple doses of intravenous interleukin 10 in steroid-refractory Crohn's disease. Crohn's Disease Study Group. Gastroenterology. 1997 Aug;113(2):383–389. doi: 10.1053/gast.1997.v113.pm9247454. [DOI] [PubMed] [Google Scholar]
- van Dullemen H. M., van Deventer S. J., Hommes D. W., Bijl H. A., Jansen J., Tytgat G. N., Woody J. Treatment of Crohn's disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2). Gastroenterology. 1995 Jul;109(1):129–135. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]