Abstract
BACKGROUND—The pharmacological inhibition of exocrine pancreatic secretion with the somatostatin analogue octreotide has been advocated as a specific treatment of acute pancreatitis. AIM—To investigate the efficacy of octreotide in acute pancreatitis in a randomised, placebo controlled trial. METHODS—302 patients from 32 hospitals, fulfilling the criteria for moderate to severe acute pancreatitis within 96 hours of the onset of symptoms, were randomly assigned to one of three treatment groups: group P (n=103) received placebo, while groups O1 (n=98) and O2 (n=101) received 100 and 200 µg of octreotide, respectively, by subcutaneous injection three times daily for seven days. The primary outcome variable was a score composed of mortality and 15 typical complications of acute pancreatitis. RESULTS—The three groups were well matched with respect to pretreatment characteristics. An intent to treat analysis of all 302 patients revealed no significant differences among treatment groups with respect to mortality (P: 16%; O1: 15%; O2: 12%), the rate of newly developed complications, the duration of pain, surgical interventions, or the length of the hospital stay. A valid for efficacy analysis (251 patients) also revealed no significant differences. CONCLUSIONS—This trial shows no benefit of octreotide in the treatment of acute pancreatitis. Keywords: acute pancreatitis; somatostatin; octreotide; randomised controlled multicentre trial
Full Text
The Full Text of this article is available as a PDF (150.7 KB).
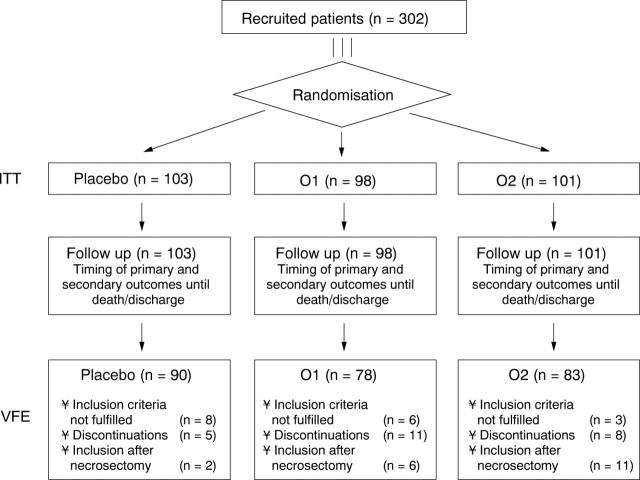
Figure 1 .
Flow chart of the allocation of patients to the study groups. ITT, intent to treat analysis; VFE, valid for efficacy analysis. Some patients were excluded for more than one reason.
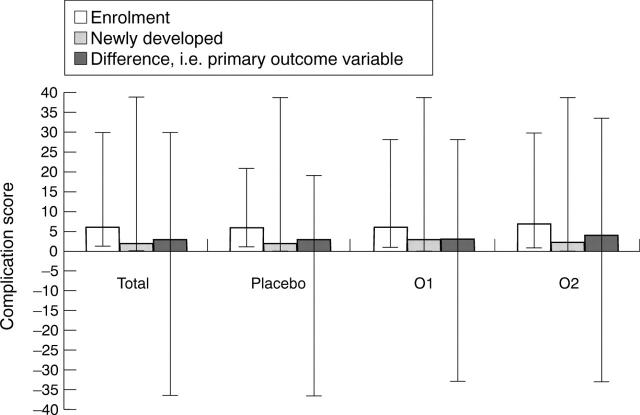
Figure 2 .
Scores for complications on enrolment, newly developed complications, and their difference in the intent to treat patient group. Results expressed as medians and ranges. The difference in complication scores (enrolment minus new complications) was computed for each patient. The median of the individual differences was then calculated.
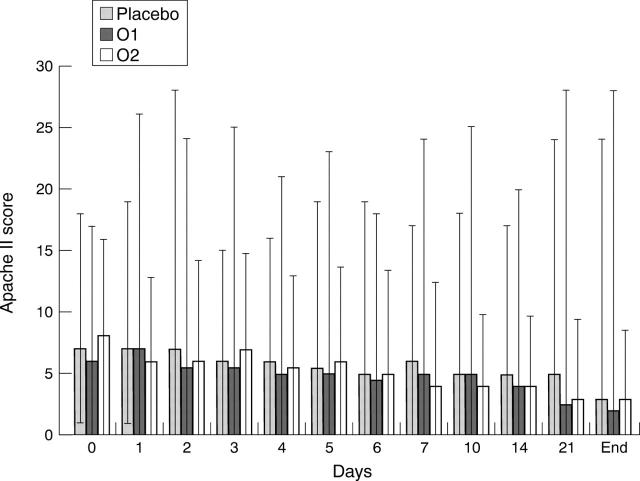
Figure 3 .
Course of the Apache II score in the intent to treat patient group. Results are expressed as medians and ranges.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augelli N. V., Hussain S. M., McKain M. M., Fietsam R., Bierema T., Fegley M., Bendick P., Villalba M., Lucas R., Glover J. L. Effect of SMS201-995 (a long-acting somatostatin analog) on bile-induced acute hemorrhagic pancreatitis in the dog. Am Surg. 1989 Jun;55(6):389–391. [PubMed] [Google Scholar]
- Bauer W., Briner U., Doepfner W., Haller R., Huguenin R., Marbach P., Petcher T. J., Pless SMS 201-995: a very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci. 1982 Sep 13;31(11):1133–1140. doi: 10.1016/0024-3205(82)90087-x. [DOI] [PubMed] [Google Scholar]
- Baxter J. N., Jenkins S. A., Day D. W., Roberts N. B., Cowell D. C., Mackie C. R., Shields R. Effects of somatostatin and a long-acting somatostatin analogue on the prevention and treatment of experimentally induced acute pancreatitis in the rat. Br J Surg. 1985 May;72(5):382–385. doi: 10.1002/bjs.1800720516. [DOI] [PubMed] [Google Scholar]
- Baxter J. N., Jenkins S. A., Day D. W., Shields R. Effects of a somatostatin analogue (SMS 201-995) on hepatic and splenic reticulo-endothelial function in the rat. Br J Surg. 1985 Dec;72(12):1005–1008. doi: 10.1002/bjs.1800721224. [DOI] [PubMed] [Google Scholar]
- Beechey-Newman N. Controlled trial of high-dose octreotide in treatment of acute pancreatitis. Evidence of improvement in disease severity. Dig Dis Sci. 1993 Apr;38(4):644–647. doi: 10.1007/BF01316794. [DOI] [PubMed] [Google Scholar]
- Beger H. G., Bittner R., Block S., Büchler M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology. 1986 Aug;91(2):433–438. doi: 10.1016/0016-5085(86)90579-2. [DOI] [PubMed] [Google Scholar]
- Beger H. G., Bittner R., Büchler M., Hess W., Schmitz J. E. Hemodynamic data pattern in patients with acute pancreatitis. Gastroenterology. 1986 Jan;90(1):74–79. doi: 10.1016/0016-5085(86)90077-6. [DOI] [PubMed] [Google Scholar]
- Beger H. G., Büchler M., Bittner R., Block S., Nevalainen T., Roscher R. Necrosectomy and postoperative local lavage in necrotizing pancreatitis. Br J Surg. 1988 Mar;75(3):207–212. doi: 10.1002/bjs.1800750306. [DOI] [PubMed] [Google Scholar]
- Begg C., Cho M., Eastwood S., Horton R., Moher D., Olkin I., Pitkin R., Rennie D., Schulz K. F., Simel D. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996 Aug 28;276(8):637–639. doi: 10.1001/jama.276.8.637. [DOI] [PubMed] [Google Scholar]
- Binder M., Uhl W., Friess H., Malfertheiner P., Büchler M. W. Octreotide in the treatment of acute pancreatitis: results of a unicenter prospective trial with three different octreotide dosages. Digestion. 1994;55 (Suppl 1):20–23. doi: 10.1159/000201184. [DOI] [PubMed] [Google Scholar]
- Bordas J. M., Toledo V., Mondelo F., Rodés J. Prevention of pancreatic reactions by bolus somatostatin administration in patients undergoing endoscopic retrograde cholangio-pancreatography and endoscopic sphincterotomy. Horm Res. 1988;29(2-3):106–108. doi: 10.1159/000180981. [DOI] [PubMed] [Google Scholar]
- Bradley E. L., 3rd A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993 May;128(5):586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- Büchler M., Friess H., Klempa I., Hermanek P., Sulkowski U., Becker H., Schafmayer A., Baca I., Lorenz D., Meister R. Role of octreotide in the prevention of postoperative complications following pancreatic resection. Am J Surg. 1992 Jan;163(1):125–131. doi: 10.1016/0002-9610(92)90264-r. [DOI] [PubMed] [Google Scholar]
- Büchler M., Malfertheiner P., Friess H., Isenmann R., Vanek E., Grimm H., Schlegel P., Friess T., Beger H. G. Human pancreatic tissue concentration of bactericidal antibiotics. Gastroenterology. 1992 Dec;103(6):1902–1908. doi: 10.1016/0016-5085(92)91450-i. [DOI] [PubMed] [Google Scholar]
- Büchler M., Malfertheiner P., Schädlich H., Nevalainen T. J., Friess H., Beger H. G. Role of phospholipase A2 in human acute pancreatitis. Gastroenterology. 1989 Dec;97(6):1521–1526. doi: 10.1016/0016-5085(89)90398-3. [DOI] [PubMed] [Google Scholar]
- Büchler M., Malfertheiner P., Uhl W., Schölmerich J., Stöckmann F., Adler G., Gaus W., Rolle K., Beger H. G. Gabexate mesilate in human acute pancreatitis. German Pancreatitis Study Group. Gastroenterology. 1993 Apr;104(4):1165–1170. doi: 10.1016/0016-5085(93)90288-n. [DOI] [PubMed] [Google Scholar]
- Choi T. K., Mok F., Zhan W. H., Fan S. T., Lai E. C., Wong J. Somatostatin in the treatment of acute pancreatitis: a prospective randomised controlled trial. Gut. 1989 Feb;30(2):223–227. doi: 10.1136/gut.30.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico D., Favia G., Biasiato R., Casaccia M., Falcone F., Fersini M., Marrano D., Napolitano F., Oliviero S., Rodolico A. The use of somatostatin in acute pancreatitis--results of a multicenter trial. Hepatogastroenterology. 1990 Feb;37(1):92–98. [PubMed] [Google Scholar]
- Degertekin H., Ertan A., Akdamar K., Yates R., Chen I., Coy D. H., Arimura A. Effects of somatostatin and a somatostatin agonist on diet-induced pancreatitis in mice. Peptides. 1985 Nov-Dec;6(6):1245–1247. doi: 10.1016/0196-9781(85)90457-7. [DOI] [PubMed] [Google Scholar]
- Domínguez-Muñoz J. E., Pieramico O., Büchler M., Malfertheiner P. Exocrine pancreatic function in the early phase of human acute pancreatitis. Scand J Gastroenterol. 1995 Feb;30(2):186–191. doi: 10.3109/00365529509093260. [DOI] [PubMed] [Google Scholar]
- Fiedler F., Jauernig G., Keim V., Richter A., Bender H. J. Octreotide treatment in patients with necrotizing pancreatitis and pulmonary failure. Intensive Care Med. 1996 Sep;22(9):909–915. doi: 10.1007/BF02044115. [DOI] [PubMed] [Google Scholar]
- Friess H., Beger H. G., Sulkowski U., Becker H., Hofbauer B., Dennler H. J., Büchler M. W. Randomized controlled multicentre study of the prevention of complications by octreotide in patients undergoing surgery for chronic pancreatitis. Br J Surg. 1995 Sep;82(9):1270–1273. doi: 10.1002/bjs.1800820938. [DOI] [PubMed] [Google Scholar]
- Gjørup I., Roikjaer O., Andersen B., Burcharth F., Hovendal C., Pedersen S. A., Christiansen P., Wara P., Andersen J. C., Balslev I. A double-blinded multicenter trial of somatostatin in the treatment of acute pancreatitis. Surg Gynecol Obstet. 1992 Nov;175(5):397–400. [PubMed] [Google Scholar]
- Isenmann R., Büchler M., Uhl W., Malfertheiner P., Martini M., Beger H. G. Pancreatic necrosis: an early finding in severe acute pancreatitis. Pancreas. 1993 May;8(3):358–361. [PubMed] [Google Scholar]
- Karimgani I., Porter K. A., Langevin R. E., Banks P. A. Prognostic factors in sterile pancreatic necrosis. Gastroenterology. 1992 Nov;103(5):1636–1640. doi: 10.1016/0016-5085(92)91189-b. [DOI] [PubMed] [Google Scholar]
- Kemmer T. P., Malfertheiner P., Büchler M., Friess H., Meschenmoser L., Ditschuneit H. Inhibition of human exocrine pancreatic secretion by the long-acting somatostatin analogue octreotide (SMS 201-995). Aliment Pharmacol Ther. 1992 Feb;6(1):41–50. doi: 10.1111/j.1365-2036.1992.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Lankisch P. G., Koop H., Winckler K., Fölsch U. R., Creutzfeldt W. Somatostatin therapy of acute experimental pancreatitis. Gut. 1977 Sep;18(9):713–716. doi: 10.1136/gut.18.9.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leese T., Holliday M., Watkins M., Thomas W. M., Neoptolemos J. P., Hall C., Attard A. A multicentre controlled clinical trial of high-volume fresh frozen plasma therapy in prognostically severe acute pancreatitis. Ann R Coll Surg Engl. 1991 Jul;73(4):207–214. [PMC free article] [PubMed] [Google Scholar]
- Limberg B., Kommerell B. Treatment of acute pancreatitis with somatostatin. N Engl J Med. 1980 Jul 31;303(5):284–284. doi: 10.1056/NEJM198007313030517. [DOI] [PubMed] [Google Scholar]
- Luengo L., Vicente V., Gris F., Coronas J. M., Escuder J., Ramón Gomez J., Castellote J. M. Influence of somatostatin in the evolution of acute pancreatitis. A prospective randomized study. Int J Pancreatol. 1994 Apr;15(2):139–144. doi: 10.1007/BF02924664. [DOI] [PubMed] [Google Scholar]
- Luiten E. J., Hop W. C., Lange J. F., Bruining H. A. Controlled clinical trial of selective decontamination for the treatment of severe acute pancreatitis. Ann Surg. 1995 Jul;222(1):57–65. doi: 10.1097/00000658-199507000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay C., Baxter J., Imrie C. A randomized, controlled trial of octreotide in the management of patients with acute pancreatitis. Int J Pancreatol. 1997 Feb;21(1):13–19. doi: 10.1007/BF02785915. [DOI] [PubMed] [Google Scholar]
- Montorsi M., Zago M., Mosca F., Capussotti L., Zotti E., Ribotta G., Fegiz G., Fissi S., Roviaro G., Peracchia A. Efficacy of octreotide in the prevention of pancreatic fistula after elective pancreatic resections: a prospective, controlled, randomized clinical trial. Surgery. 1995 Jan;117(1):26–31. doi: 10.1016/s0039-6060(05)80225-9. [DOI] [PubMed] [Google Scholar]
- Murayama K. M., Drew J. B., Joehl R. J. Does somatostatin analogue prevent experimental acute pancreatitis? Arch Surg. 1990 Dec;125(12):1570–1572. doi: 10.1001/archsurg.1990.01410240048011. [DOI] [PubMed] [Google Scholar]
- Niederau C., Niederau M., Lüthen R., Strohmeyer G., Ferrell L. D., Grendell J. H. Pancreatic exocrine secretion in acute experimental pancreatitis. Gastroenterology. 1990 Oct;99(4):1120–1127. doi: 10.1016/0016-5085(90)90633-c. [DOI] [PubMed] [Google Scholar]
- Paran H., Neufeld D., Mayo A., Shwartz I., Singer P., Kaplan O., Skornik Y., Klausner J., Freund U. Preliminary report of a prospective randomized study of octreotide in the treatment of severe acute pancreatitis. J Am Coll Surg. 1995 Aug;181(2):121–124. [PubMed] [Google Scholar]
- Pederzoli P., Bassi C., Falconi M., Camboni M. G. Efficacy of octreotide in the prevention of complications of elective pancreatic surgery. Italian Study Group. Br J Surg. 1994 Feb;81(2):265–269. doi: 10.1002/bjs.1800810237. [DOI] [PubMed] [Google Scholar]
- Pederzoli P., Bassi C., Vesentini S., Campedelli A. A randomized multicenter clinical trial of antibiotic prophylaxis of septic complications in acute necrotizing pancreatitis with imipenem. Surg Gynecol Obstet. 1993 May;176(5):480–483. [PubMed] [Google Scholar]
- Ranson J. H., Balthazar E., Caccavale R., Cooper M. Computed tomography and the prediction of pancreatic abscess in acute pancreatitis. Ann Surg. 1985 May;201(5):656–665. doi: 10.1097/00000658-198505000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner D. W., Legermate D. A., Lee M. J., Mueller P. R., Warshaw A. L. Early surgical débridement of symptomatic pancreatic necrosis is beneficial irrespective of infection. Am J Surg. 1992 Jan;163(1):105–110. doi: 10.1016/0002-9610(92)90261-o. [DOI] [PubMed] [Google Scholar]
- Regan P. T., Malagelada J. R., Go V. L., Wolf A. M., DiMagno E. P. A prospective study of the antisecretory and therapeutic effects of cimetidine and glucagon in human acute pancreatitis. Mayo Clin Proc. 1981 Aug;56(8):499–503. [PubMed] [Google Scholar]
- Sainio V., Kemppainen E., Puolakkainen P., Taavitsainen M., Kivisaari L., Valtonen V., Haapiainen R., Schröder T., Kivilaakso E. Early antibiotic treatment in acute necrotising pancreatitis. Lancet. 1995 Sep 9;346(8976):663–667. doi: 10.1016/s0140-6736(95)92280-6. [DOI] [PubMed] [Google Scholar]
- Schlarman D. E., Beinfeld M. C., Andrus C., Kaminski D. L. Effects of somatostatin on acute canine experimental pancreatitis. Int J Pancreatol. 1987 Aug;2(4):247–255. doi: 10.1007/BF02788402. [DOI] [PubMed] [Google Scholar]
- Schwedes U., Althoff P. H., Klempa I., Leuschner U., Mothes L., Raptis S., Wdowinski J., Usadel K. H. Effect of somatostatin on bile-induced acute hemorrhagic pancreatitis in the dog. Horm Metab Res. 1979 Dec;11(12):655–661. doi: 10.1055/s-0028-1092793. [DOI] [PubMed] [Google Scholar]
- Steinberg W. M., Schlesselman S. E. Treatment of acute pancreatitis. Comparison of animal and human studies. Gastroenterology. 1987 Dec;93(6):1420–1427. doi: 10.1016/0016-5085(87)90275-7. [DOI] [PubMed] [Google Scholar]
- Stratta R. J., Taylor R. J., Lowell J. A., Bynon J. S., Cattral M., Langnas A. N., Shaw B. W., Jr Selective use of Sandostatin in vascularized pancreas transplantation. Am J Surg. 1993 Dec;166(6):598–605. doi: 10.1016/s0002-9610(05)80663-4. [DOI] [PubMed] [Google Scholar]
- Uhl W., Isenmann R., Curti G., Vogel R., Beger H. G., Büchler M. W. Influence of etiology on the course and outcome of acute pancreatitis. Pancreas. 1996 Nov;13(4):335–343. doi: 10.1097/00006676-199611000-00002. [DOI] [PubMed] [Google Scholar]
- Zhu Z. H., Holt S., el-Lbishi M. S., Grady T., Taylor T. V., Powers R. E. A somatostatin analogue is protective against retrograde bile salt-induced pancreatitis in the rat. Pancreas. 1991 Sep;6(5):609–613. doi: 10.1097/00006676-199109000-00016. [DOI] [PubMed] [Google Scholar]