Abstract
BACKGROUND/AIMS—Chronic pancreatitis is an inflammatory disease of the exocrine pancreas associated with extensive fibrosis, enlarged pancreatic ducts, acinar cell degeneration, and the formation of tubular complexes. The molecular and biochemical alterations associated with these histological changes are not kown. Generally, the new family of TFF peptides (formerly known as P-domain peptides or trefoil factors) is aberrantly expressed during chronic inflammatory diseases of the gastrointestinal tract. METHODS—Using human pancreatic tissues obtained from patients with chronic pancreatitis and murine pancreatic tissues obtained from transgenic mice overexpressing transforming growth factor α (TGF-α), the expression and cellular distribution of TFF1 was analysed using northern blot analysis, polymerase chain reaction (PCR), and immunohistochemistry. RESULTS—In the normal human pancreas, TFF1 was scarce, with only a few ducts exhibiting cytoplasmic TFF1 immunoreactivity. In contrast, human chronic pancreatitis tissue specimens exhibited strong TFF1 immunoreactivity in ductal cells, areas of ductal hyperplasia, and tubular complexes. Semiquantitative PCR analysis of TFF1 mRNA levels showed enhanced expression of TFF1 in the pancreas of patients with chronic pancreatitis. Furthermore, TFF1 mRNA levels were detectable in the pancreas in four of five transgenic mice overexpressing TGF-α. In contrast, four of five wild type mice did not exhibit a TFF1 mRNA transcript. In addition, while no specific TFF1 immunoreactivity was present in the pancreas of the wild type mice, ductal epithelial cells and duct-like tubular complexes in the pancreas of the transgenic mice overexpressing TGF-α exhibited pronounced TFF1 immunoreactivity. CONCLUSIONS—Ductal cells and tubular complexes in pancreatic fibrosis express TFF1. As the 5'-flanking region of TFF1 contains an epidermal growth factor responsive enhancer region and the expression of epidermal growth factor and TGF-α is enhanced in pancreatic fibrosis, the enhanced expression of TFF1 in pancreatic fibrosis may be mediated by TGF-α. Keywords: fibrosis; pS2; pancreatitis; expression; transforming growth factor α; TFF peptides
Full Text
The Full Text of this article is available as a PDF (199.1 KB).
Figure 1 .
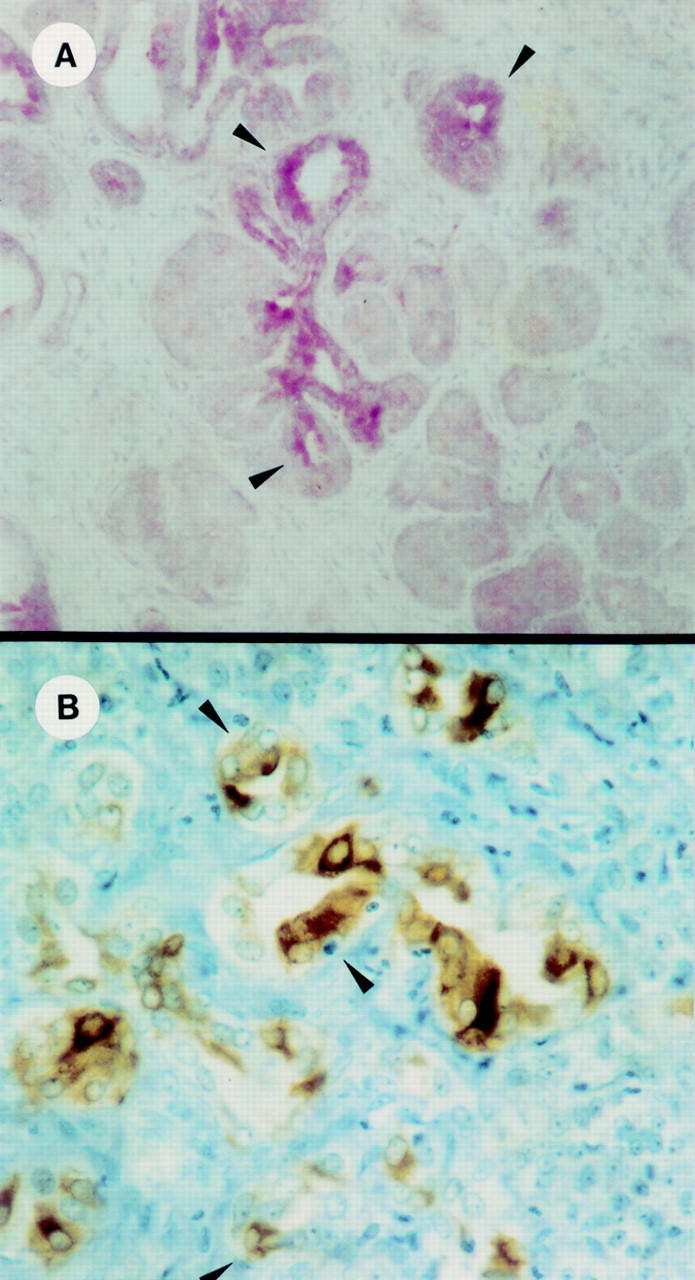
TFF1 immunoreactivity in tubular complexes in pancreatic fibrosis. (A) Transgenic mice overexpressing transforming growth factor α. Strong TFF1 immunoreactivity was observed in the duct-like cells of tubular complexes (arrowheads). (B) Human chronic pancreatitis. TFF1 immunoreactivity was also present at the surface of these duct-like cells forming a central lumen (arrowheads). Original magnifications: A, 120 ×; B, 400 ×.
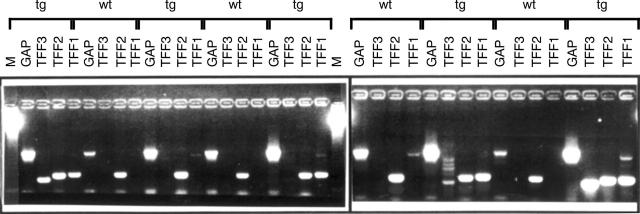
Figure 2 .
Reverse transcription polymerase chain reaction (PCR) analysis of TFF1 expression in the pancreas of wild type and transgenic mice. With cDNAs obtained from total RNA which had been extracted from the pancreas of wild type mice (wt; n = 4) and mice overexpressing transforming growth factor α (tg; n = 5), a band corresponding to TFF1 was observed in the pancreas in four of five transgenic mice. In contrast, only one wild type mouse exhibited the TFF1 transcript in the pancreas. TFF2 was expressed in all transgenic and wild type mice, whereas TFF3 expression was detectable in three of five transgenic mice. Parallel PCR amplification of the cDNAs using two primers specific for glyceraldehyde-3-phosphate dehydrogenase (GAP) confirmed the integrity of the cDNAs used. M, DNA marker.
Figure 3 .
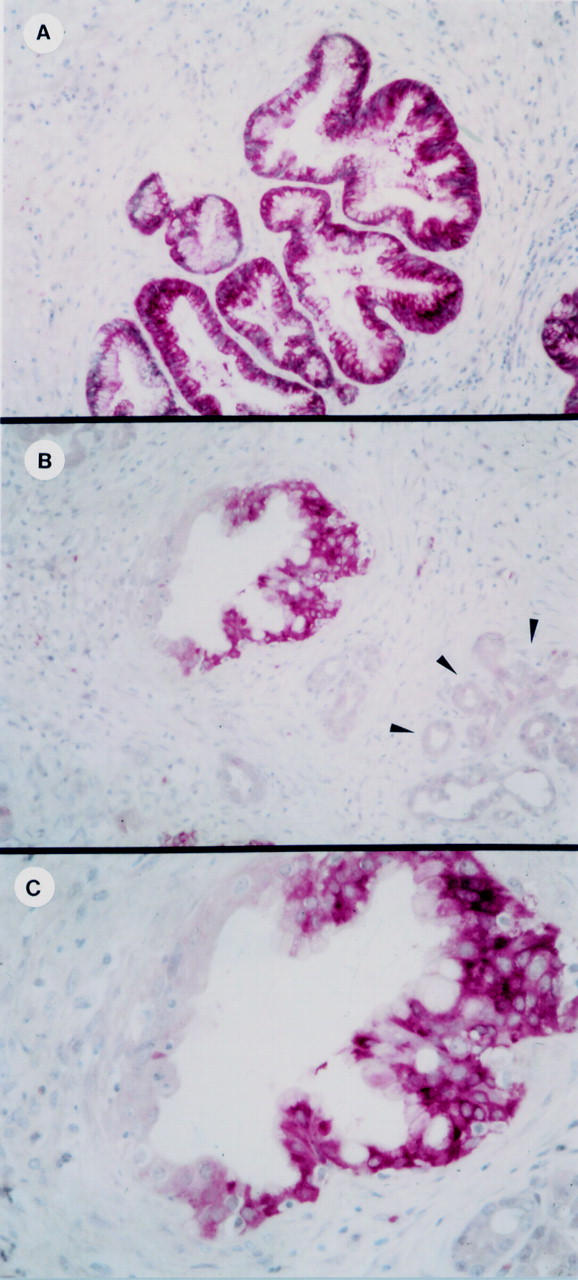
TFF1 immunoreactivity in chronic pancreatitis. Strong TFF1 immunoreactivity was present in the vast majority of pancreatic ducts (A) and areas of ductal hyperplasia also exhibited intense TFF1 immunoreactivity (B,C). However, not all ducts exhibited TFF1 immunoreactivity (B, arrowheads), and acinar and islet cells, as well as fibroblasts and inflammatory cells, were generally devoid of TFF1 immunoreactivity. Original magnifications: A, B, 120 ×; C, 400 ×.
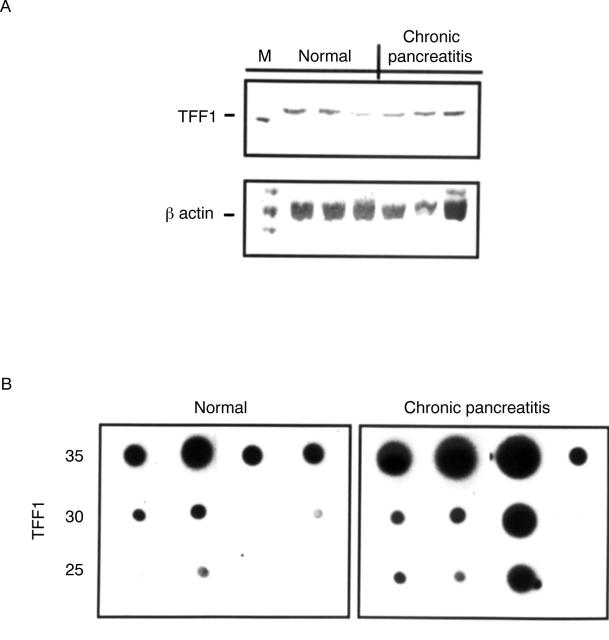
Figure 4 .
(A) Reverse transcription polymerase chain reaction (PCR) analysis of TFF1 expression in the normal pancreas and in chronic pancreatitis. With cDNA obtained from total RNA extracted from normal pancreatic tissue samples (n = 3) and samples from patients with chronic pancreatitis (n =3), a band corresponding to TFF1 was present in all samples, as detected by polyacrylamide gel electrophoresis. Parallel PCR amplification of the cDNAs using two primers specific for β-actin confirmed the integrity of the cDNAs used. M, DNA marker. (B) Semiquantitative PCR analysis of TFF1 expression in chronic pancreatitis. Total RNA was extracted as previously described and PCR analysis was performed. A 15 µl portion of the amplification mixture was removed every five cycles from cycles 25 to 35. The samples were dot-blotted to Nylon membranes and probed using a [32P]dCTP random prime labelled TFF1 cDNA probe.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias A. E., Bendayan M. Differentiation of pancreatic acinar cells into duct-like cells in vitro. Lab Invest. 1993 Nov;69(5):518–530. [PubMed] [Google Scholar]
- Balmain A., Krumlauf R., Vass J. K., Birnie G. D. Cloning and characterisation of the abundant cytoplasmic 7S RNA from mouse cells. Nucleic Acids Res. 1982 Jul 24;10(14):4259–4277. doi: 10.1093/nar/10.14.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockman D. E., Boydston W. R., Anderson M. C. Origin of tubular complexes in human chronic pancreatitis. Am J Surg. 1982 Aug;144(2):243–249. doi: 10.1016/0002-9610(82)90518-9. [DOI] [PubMed] [Google Scholar]
- Bockman D. E., Merlino G. Cytological changes in the pancreas of transgenic mice overexpressing transforming growth factor alpha. Gastroenterology. 1992 Dec;103(6):1883–1892. doi: 10.1016/0016-5085(92)91448-d. [DOI] [PubMed] [Google Scholar]
- Chinery R., Playford R. J. Combined intestinal trefoil factor and epidermal growth factor is prophylactic against indomethacin-induced gastric damage in the rat. Clin Sci (Lond) 1995 Apr;88(4):401–403. doi: 10.1042/cs0880401. [DOI] [PubMed] [Google Scholar]
- Collier J. D., Bennett M. K., Bassendine M. F., Lendrum R. Immunolocalization of pS2, a putative growth factor, in pancreatic carcinoma. J Gastroenterol Hepatol. 1995 Jul-Aug;10(4):396–400. doi: 10.1111/j.1440-1746.1995.tb01590.x. [DOI] [PubMed] [Google Scholar]
- Cook G. A., Yeomans N. D., Giraud A. S. Temporal expression of trefoil peptides in the TGF-alpha knockout mouse after gastric ulceration. Am J Physiol. 1997 Jun;272(6 Pt 1):G1540–G1549. doi: 10.1152/ajpgi.1997.272.6.G1540. [DOI] [PubMed] [Google Scholar]
- Dallman M. J., Larsen C. P., Morris P. J. Cytokine gene transcription in vascularised organ grafts: analysis using semiquantitative polymerase chain reaction. J Exp Med. 1991 Aug 1;174(2):493–496. doi: 10.1084/jem.174.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dante R., Ribieras S., Baldassini S., Martin V., Benzerara O., Bouteille C., Brémond A., Frappart L., Rio M. C., Lasne Y. Expression of an estrogen-induced breast cancer-associated protein (pS2) in benign and malignant human ovarian cysts. Lab Invest. 1994 Aug;71(2):188–192. [PubMed] [Google Scholar]
- Dignass A., Lynch-Devaney K., Kindon H., Thim L., Podolsky D. K. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994 Jul;94(1):376–383. doi: 10.1172/JCI117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert M., Kasper H. U., Hernberg S., Friess H., Büchler M. W., Roessner A., Korc M., Malfertheiner P. Overexpression of platelet-derived growth factor (PDGF) B chain and type beta PDGF receptor in human chronic pancreatitis. Dig Dis Sci. 1998 Mar;43(3):567–574. doi: 10.1023/a:1018867209170. [DOI] [PubMed] [Google Scholar]
- Ebert M., Yokoyama M., Kobrin M. S., Friess H., Lopez M. E., Büchler M. W., Johnson G. R., Korc M. Induction and expression of amphiregulin in human pancreatic cancer. Cancer Res. 1994 Aug 1;54(15):3959–3962. [PubMed] [Google Scholar]
- Elsässer H. P., Adler G., Kern H. F. Fibroblast structure and function during regeneration from hormone-induced acute pancreatitis in the rat. Pancreas. 1989;4(2):169–178. doi: 10.1097/00006676-198904000-00005. [DOI] [PubMed] [Google Scholar]
- Friess H., Yamanaka Y., Büchler M., Beger H. G., Do D. A., Kobrin M. S., Korc M. Increased expression of acidic and basic fibroblast growth factors in chronic pancreatitis. Am J Pathol. 1994 Jan;144(1):117–128. [PMC free article] [PubMed] [Google Scholar]
- Friess H., Yamanaka Y., Büchler M., Hammer K., Kobrin M. S., Beger H. G., Korc M. A subgroup of patients with chronic pancreatitis overexpress the c-erb B-2 protooncogene. Ann Surg. 1994 Aug;220(2):183–192. doi: 10.1097/00000658-199408000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenring J. R., Poulsom R., Ray G. S., Wright N., Meise K. S., Coffey R. J., Jr Expression of trefoil peptides in the gastric mucosa of transgenic mice overexpressing transforming growth factor-alpha. Growth Factors. 1996;13(1-2):111–119. doi: 10.3109/08977199609034571. [DOI] [PubMed] [Google Scholar]
- Henry J. A., Bennett M. K., Piggott N. H., Levett D. L., May F. E., Westley B. R. Expression of the pNR-2/pS2 protein in diverse human epithelial tumours. Br J Cancer. 1991 Oct;64(4):677–682. doi: 10.1038/bjc.1991.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J. A., Piggott N. H., Mallick U. K., Nicholson S., Farndon J. R., Westley B. R., May F. E. pNR-2/pS2 immunohistochemical staining in breast cancer: correlation with prognostic factors and endocrine response. Br J Cancer. 1991 Apr;63(4):615–622. doi: 10.1038/bjc.1991.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann W., Hauser F. The P-domain or trefoil motif: a role in renewal and pathology of mucous epithelia? Trends Biochem Sci. 1993 Jul;18(7):239–243. doi: 10.1016/0968-0004(93)90170-r. [DOI] [PubMed] [Google Scholar]
- Jakowlew S. B., Breathnach R., Jeltsch J. M., Masiakowski P., Chambon P. Sequence of the pS2 mRNA induced by estrogen in the human breast cancer cell line MCF-7. Nucleic Acids Res. 1984 Mar 26;12(6):2861–2878. doi: 10.1093/nar/12.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch J. M., Roberts M., Schatz C., Garnier J. M., Brown A. M., Chambon P. Structure of the human oestrogen-responsive gene pS2. Nucleic Acids Res. 1987 Feb 25;15(4):1401–1414. doi: 10.1093/nar/15.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöppel G., Maillet B. Pathology of acute and chronic pancreatitis. Pancreas. 1993 Nov;8(6):659–670. doi: 10.1097/00006676-199311000-00001. [DOI] [PubMed] [Google Scholar]
- Korc M., Friess H., Yamanaka Y., Kobrin M. S., Buchler M., Beger H. G. Chronic pancreatitis is associated with increased concentrations of epidermal growth factor receptor, transforming growth factor alpha, and phospholipase C gamma. Gut. 1994 Oct;35(10):1468–1473. doi: 10.1136/gut.35.10.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre O., Chenard M. P., Masson R., Linares J., Dierich A., LeMeur M., Wendling C., Tomasetto C., Chambon P., Rio M. C. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science. 1996 Oct 11;274(5285):259–262. doi: 10.1126/science.274.5285.259. [DOI] [PubMed] [Google Scholar]
- Lefebvre O., Wolf C., Kédinger M., Chenard M. P., Tomasetto C., Chambon P., Rio M. C. The mouse one P-domain (pS2) and two P-domain (mSP) genes exhibit distinct patterns of expression. J Cell Biol. 1993 Jul;122(1):191–198. doi: 10.1083/jcb.122.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez A. M., Berry M., Imler J. L., Chambon P. The 5' flanking region of the pS2 gene contains a complex enhancer region responsive to oestrogens, epidermal growth factor, a tumour promoter (TPA), the c-Ha-ras oncoprotein and the c-jun protein. EMBO J. 1989 Mar;8(3):823–829. doi: 10.1002/j.1460-2075.1989.tb03443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa I., Longnecker D. S., Scarpelli D. G., Pour P., Reddy J. K., Lefkowitz M. Ductal metaplasia of human exocrine pancreas and its association with carcinoma. Cancer Res. 1985 Mar;45(3):1285–1290. [PubMed] [Google Scholar]
- Piggott N. H., Henry J. A., May F. E., Westley B. R. Antipeptide antibodies against the pNR-2 oestrogen-regulated protein of human breast cancer cells and detection of pNR-2 expression in normal tissues by immunohistochemistry. J Pathol. 1991 Feb;163(2):95–104. doi: 10.1002/path.1711630204. [DOI] [PubMed] [Google Scholar]
- Playford R. J., Marchbank T., Chinery R., Evison R., Pignatelli M., Boulton R. A., Thim L., Hanby A. M. Human spasmolytic polypeptide is a cytoprotective agent that stimulates cell migration. Gastroenterology. 1995 Jan;108(1):108–116. doi: 10.1016/0016-5085(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Sandgren E. P., Luetteke N. C., Palmiter R. D., Brinster R. L., Lee D. C. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell. 1990 Jun 15;61(6):1121–1135. doi: 10.1016/0092-8674(90)90075-p. [DOI] [PubMed] [Google Scholar]
- Sands B. E., Podolsky D. K. The trefoil peptide family. Annu Rev Physiol. 1996;58:253–273. doi: 10.1146/annurev.ph.58.030196.001345. [DOI] [PubMed] [Google Scholar]
- Thim L. Trefoil peptides: from structure to function. Cell Mol Life Sci. 1997 Dec;53(11-12):888–903. doi: 10.1007/s000180050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M., Lührs H., Klöppel G., Adler G., Schmid R. M. Malignant transformation of duct-like cells originating from acini in transforming growth factor transgenic mice. Gastroenterology. 1998 Nov;115(5):1254–1262. doi: 10.1016/s0016-5085(98)70098-8. [DOI] [PubMed] [Google Scholar]
- Welter C., Theisinger B., Seitz G., Tomasetto C., Rio M. C., Chambon P., Blin N. Association of the human spasmolytic polypeptide and an estrogen-induced breast cancer protein (pS2) with human pancreatic carcinoma. Lab Invest. 1992 Feb;66(2):187–192. [PubMed] [Google Scholar]
- Williams G. R., Wright N. A. Trefoil factor family domain peptides. Virchows Arch. 1997 Nov;431(5):299–304. doi: 10.1007/s004280050102. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Hoffmann W., Otto W. R., Rio M. C., Thim L. Rolling in the clover: trefoil factor family (TFF)-domain peptides, cell migration and cancer. FEBS Lett. 1997 May 19;408(2):121–123. doi: 10.1016/s0014-5793(97)00424-9. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Pike C., Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990 Jan 4;343(6253):82–85. doi: 10.1038/343082a0. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Poulsom R., Stamp G. W., Hall P. A., Jeffery R. E., Longcroft J. M., Rio M. C., Tomasetto C., Chambon P. Epidermal growth factor (EGF/URO) induces expression of regulatory peptides in damaged human gastrointestinal tissues. J Pathol. 1990 Dec;162(4):279–284. doi: 10.1002/path.1711620402. [DOI] [PubMed] [Google Scholar]