Abstract
BACKGROUND—The molecular mechanisms underlying the differential sensitivity of human colon carcinoma cells to retinoid mediated growth inhibition are poorly understood. AIM—To identify the intracellular mechanisms responsible for resistance against retinoid mediated growth inhibition in human colon carcinoma cells. METHODS—Anchorage independent growth of the human colon carcinoma cell lines HT29 and LoVo was determined by a human tumour clonogenic assay. Retinoid receptor expression was evaluated by reverse transcription polymerase chain reaction and northern blotting. Retinoid mediated transactivation was assessed by transient transfection of a pTK::βREx2-luc reporter construct. Retinoid receptor overexpression was achieved by selecting stably transfected cell clones. RESULTS—Retinoid treatment resulted in profound dose dependent growth inhibition in HT29 cells, while LoVo cells were unaffected. The two cell lines express identical patterns of nuclear retinoid receptor mRNA transcripts. However, on retinoid treatment, retinoic acid receptor β gene expression was upregulated only in retinoid sensitive HT29 cells, but not in retinoid resistant LoVo cells. In accordance, stable overexpression of retinoic acid receptor β but not α or γ conferred retinoid mediated growth inhibition on LoVo cells. CONCLUSION—Induction of retinoic acid receptor β expression is required and sufficent to confer retinoid mediated growth inhibition on human colon carcinoma cells. Keywords: colon cancer; retinoic acid; retinoic acid receptor
Full Text
The Full Text of this article is available as a PDF (197.9 KB).
Figure 1 .
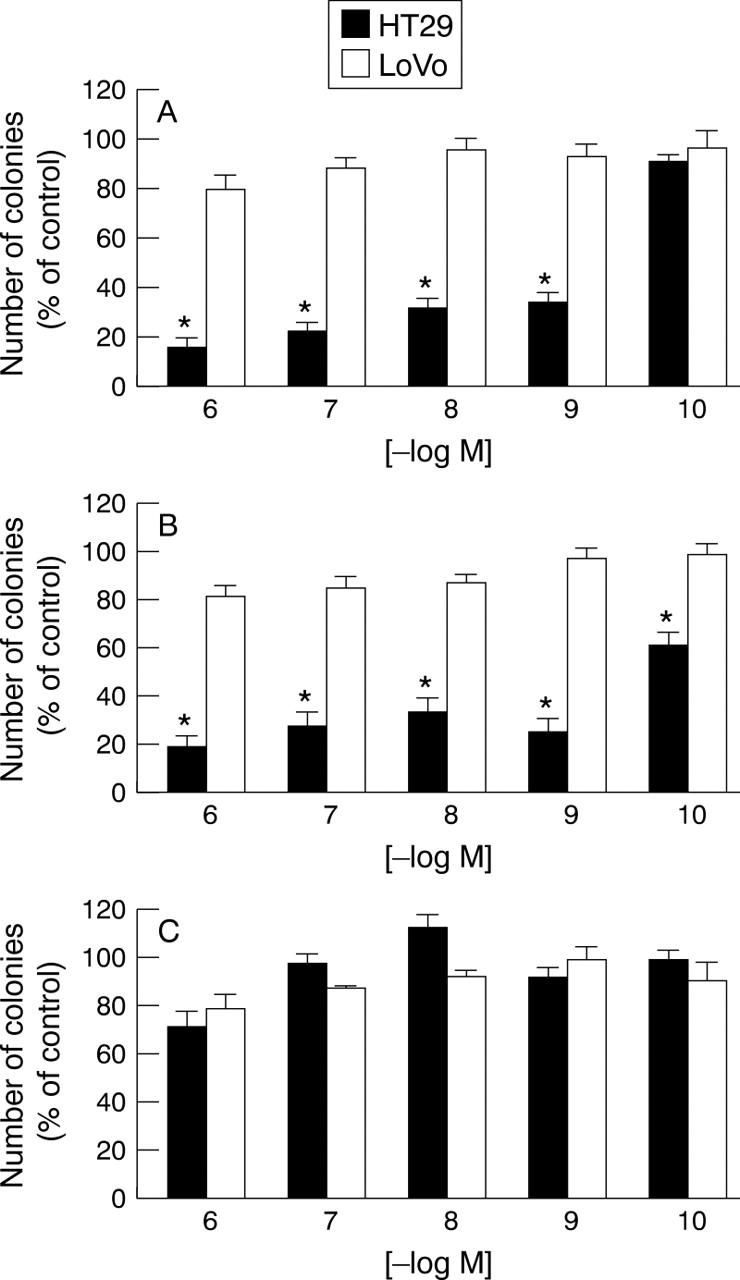
Differential effects of retinoid derivatives on anchorage independent growth. HT29 and LoVo cells were analysed using the human tumour clonogenic assay with the indicated final concentrations of each retinoid analogue (A, all-trans-retinoic acid; B, 13-cis-retinoic acid; C, 9-cis-retinoic acid). After 10 days the number of colonies was evaluated and expressed as percentage of vehicle treated controls. Values are mean (SEM) from three independent experiments, each performed in triplicate. *p<0.05 v controls.
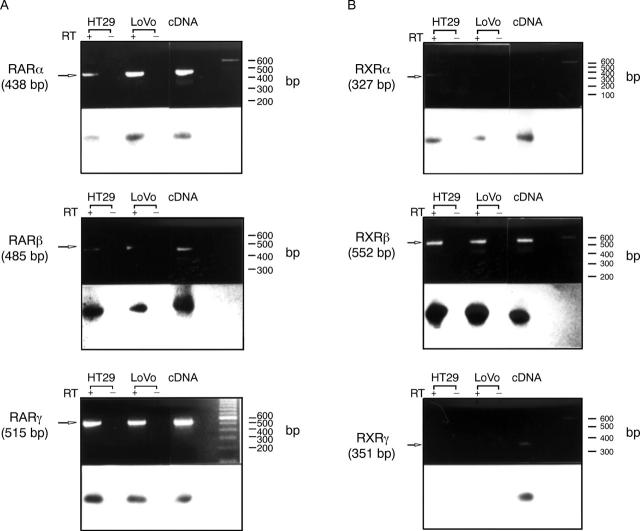
Figure 2 .
Identical expression of nuclear retinoid receptors in HT29 and LoVo cells. Reverse transcription polymerase chain reaction (RT-PCR) analysis was performed on HT29 and LoVo cells using receptor specific oligonucleotide primers. The respective receptor subtype cDNA was used as a positive internal control. RT-PCR with (+) or without (−) prior reverse transcription (the latter served as a negative internal control) was performed. Duplicate aliquots from each RT-PCR product were electrophoresed through a 1% agarose gel and then subjected to Southern blot analysis using the respective cDNA probes for the retinoic acid receptors (RARs)/retinoid X receptors (RXRs) to ensure specificity of the amplification product. Size determination was performed using a DNA ladder. (A) RT-PCR for RARs; (B) RT-PCR for RXRs.
Figure 3 .
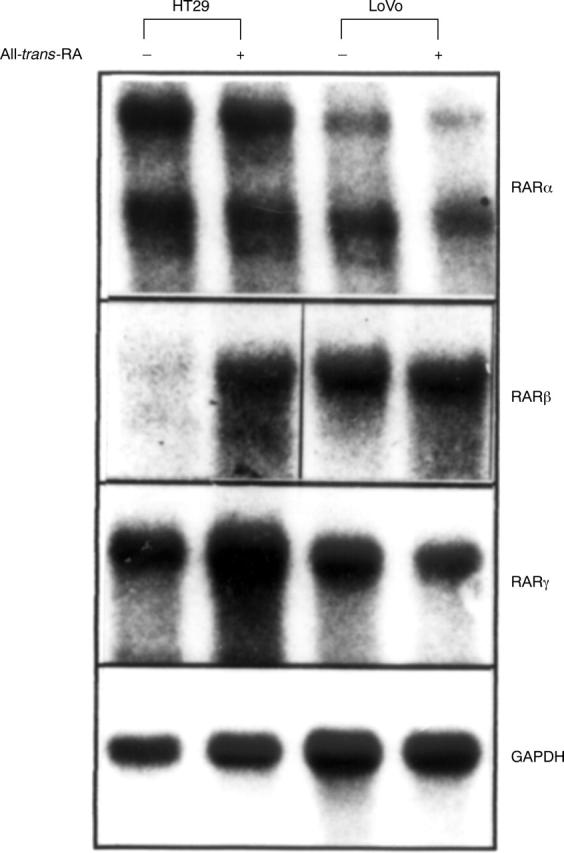
Differential autoregulation of retinoic acid receptor (RAR)β by retinoids. HT29 and LoVo cells were incubated with vehicle or 10 µM all-trans-retinoic acid (all-trans RA) for 24 hours. Then 25 µg total RNA was analysed by northern blotting using radioactively labelled cDNAs specific for the RAR subtypes. The blots were then stripped and rehybridised with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA to ensure equal RNA loading. The results shown are representative of three independent experiments.
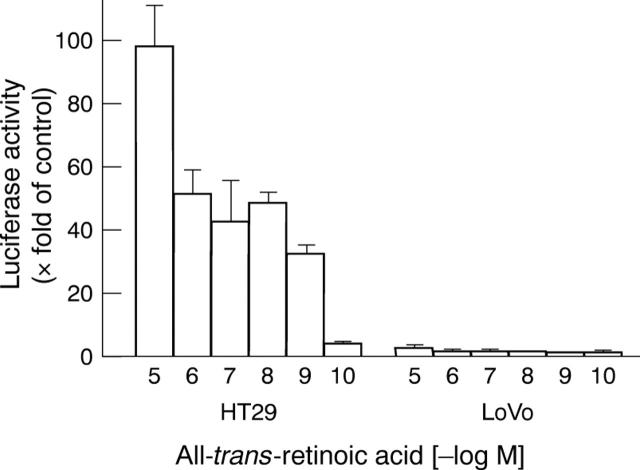
Figure 4 .
Differential effects of all-trans-retinoic acid on transactivation. HT29 and LoVo cells were transiently transfected with the pTK::βREx2-luc reporter construct. After incubation with the indicated concentrations of all-trans RA for 24 hours, luciferase activity was determined and expressed as fold increase over basal (luciferase activity in the absence of retinoids). Values are mean (SEM) from six independent experiments, each performed in triplicate wells.
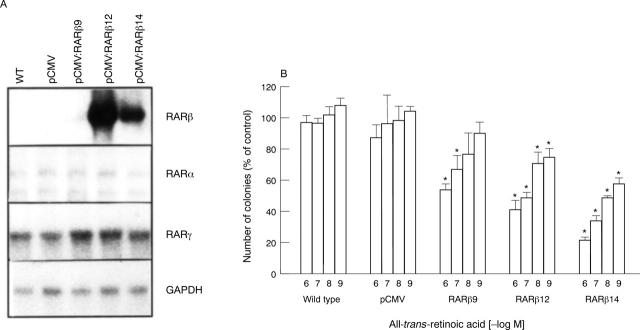
Figure 5 .
Overexpression of retinoic acid receptor (RAR)β restores retinoid sensitivity in LoVo cells. After stable transfection with the RARβ cDNA, overexpression of RARβ mRNA was confirmed by northern blotting (A). GAPDH, Glyceraldehyde-3-phosphate dehydrogenase. Transfected (pCMV:RARβ9, pCMV:RARβ12, pCMV:RARβ14), mock transfected (pCMV), and wild type (WT) LoVo cells were then analysed by human tumour clonogenic assay using the indicated concentrations of all-trans-retinoic acid. (B) After 10 days the number of colonies was evaluated and expressed as a percentage of vehicle treated contols. Results shown are mean (SEM) from three independent experiments, each performed in triplicate. *p<0.05 v controls.
Figure 6 .
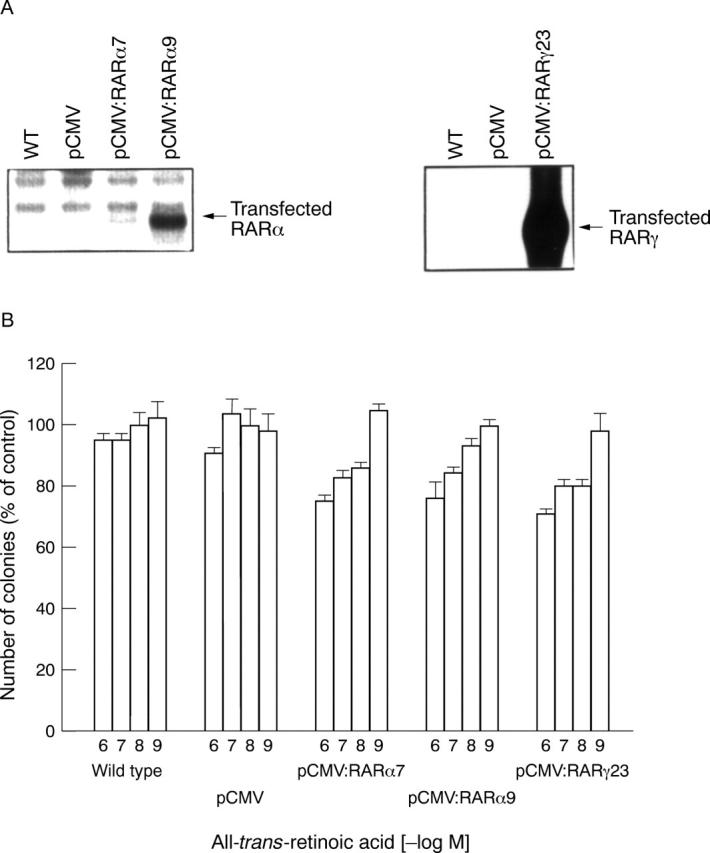
Overexpression of retinoic acid receptor (RAR)α or γ does not restore retinoid sensitivity in LoVo cells. After stable transfection with the RARα and γ cDNA, overexpression of the respective RAR subtype mRNA was confirmed by northern blotting (A). Transfected (pCMV:RARα7, pCMV:RARα9, pCMV:RARγ23), mock transfected (pCMV), and wild type (WT) LoVo cells were then analysed by human tumour clonogenic assay using the indicated concentrations of all-trans-retinoic acid. (B) After 10 days the number of colonies was evaluated and expressed as a percentage of vehicle treated contols. Results shown are mean (SEM) from three independent experiments, each performed in triplicate.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkenstam A., Vivanco Ruiz M. M., Barettino D., Horikoshi M., Stunnenberg H. G. Cooperativity in transactivation between retinoic acid receptor and TFIID requires an activity analogous to E1A. Cell. 1992 May 1;69(3):401–412. doi: 10.1016/0092-8674(92)90443-g. [DOI] [PubMed] [Google Scholar]
- Brand N., Petkovich M., Krust A., Chambon P., de Thé H., Marchio A., Tiollais P., Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988 Apr 28;332(6167):850–853. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- Davis K. D., Lazar M. A. Induction of retinoic acid receptor-beta by retinoic acid is cell specific. Endocrinology. 1993 Apr;132(4):1469–1474. doi: 10.1210/endo.132.4.8384988. [DOI] [PubMed] [Google Scholar]
- Dollé P., Ruberte E., Leroy P., Morriss-Kay G., Chambon P. Retinoic acid receptors and cellular retinoid binding proteins. I. A systematic study of their differential pattern of transcription during mouse organogenesis. Development. 1990 Dec;110(4):1133–1151. doi: 10.1242/dev.110.4.1133. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Forman B. M., Umesono K., Chen J., Evans R. M. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995 May 19;81(4):541–550. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Giguère V. Retinoic acid receptors and cellular retinoid binding proteins: complex interplay in retinoid signaling. Endocr Rev. 1994 Feb;15(1):61–79. doi: 10.1210/edrv-15-1-61. [DOI] [PubMed] [Google Scholar]
- Heyman R. A., Mangelsdorf D. J., Dyck J. A., Stein R. B., Eichele G., Evans R. M., Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992 Jan 24;68(2):397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- Kane K. F., Langman M. J., Williams G. R. Antiproliferative responses to two human colon cancer cell lines to vitamin D3 are differently modified by 9-cis-retinoic acid. Cancer Res. 1996 Feb 1;56(3):623–632. [PubMed] [Google Scholar]
- Kawamori T., Tanaka T., Suzui M., Okamoto K., Tamai Y., Torihara M., Yamahara J., Mori H. Chemoprevention of azoxymethane-induced intestinal carcinogenesis by a novel synthesized retinoidal butenolide, 5-hydroxy-4-(2-phenyl-(E)-ethenyl)-2(5H)-furanone, in rats. Carcinogenesis. 1995 Apr;16(4):795–800. doi: 10.1093/carcin/16.4.795. [DOI] [PubMed] [Google Scholar]
- Kruyt F. A., Folkers G. E., Walhout A. J., van der Leede B. J., van der Saag P. T. E1A functions as a coactivator of retinoic acid-dependent retinoic acid receptor-beta 2 promoter activation. Mol Endocrinol. 1993 Apr;7(4):604–615. doi: 10.1210/mend.7.4.8389000. [DOI] [PubMed] [Google Scholar]
- Lee X., Si S. P., Tsou H. C., Peacocke M. Cellular aging and transformation suppression: a role for retinoic acid receptor beta 2. Exp Cell Res. 1995 May;218(1):296–304. doi: 10.1006/excr.1995.1158. [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner P., Chambon P. Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem Sci. 1992 Oct;17(10):427–433. doi: 10.1016/0968-0004(92)90014-z. [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Liepkalns V. A., Icard-Liepkalns C. Retinoic-acid-induced augmentation of molecular species carrying sialosyl Lewis(a) antigen in colorectal-carcinoma cell cultures. Int J Cancer. 1993 Sep 9;55(2):256–261. doi: 10.1002/ijc.2910550215. [DOI] [PubMed] [Google Scholar]
- Lippman S. M., Heyman R. A., Kurie J. M., Benner S. E., Hong W. K. Retinoids and chemoprevention: clinical and basic studies. J Cell Biochem Suppl. 1995;22:1–10. doi: 10.1002/jcb.240590802. [DOI] [PubMed] [Google Scholar]
- Marshall G. M., Cheung B., Stacey K. P., Camacho M. L., Simpson A. M., Kwan E., Smith S., Haber M., Norris M. D. Increased retinoic acid receptor gamma expression suppresses the malignant phenotype and alters the differentiation potential of human neuroblastoma cells. Oncogene. 1995 Aug 3;11(3):485–491. [PubMed] [Google Scholar]
- Minucci S., Zand D. J., Dey A., Marks M. S., Nagata T., Grippo J. F., Ozato K. Dominant negative retinoid X receptor beta inhibits retinoic acid-responsive gene regulation in embryonal carcinoma cells. Mol Cell Biol. 1994 Jan;14(1):360–372. doi: 10.1128/mcb.14.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moasser M. M., DeBlasio A., Dmitrovsky E. Response and resistance to retinoic acid are mediated through the retinoic acid nuclear receptor gamma in human teratocarcinomas. Oncogene. 1994 Mar;9(3):833–840. [PubMed] [Google Scholar]
- Muindi J. R., Frankel S. R., Huselton C., DeGrazia F., Garland W. A., Young C. W., Warrell R. P., Jr Clinical pharmacology of oral all-trans retinoic acid in patients with acute promyelocytic leukemia. Cancer Res. 1992 Apr 15;52(8):2138–2142. [PubMed] [Google Scholar]
- Niles R. M., Wilhelm S. A., Thomas P., Zamcheck N. The effect of sodium butyrate and retinoic acid on growth and CEA production in a series of human colorectal tumor cell lines representing different states of differentiation. Cancer Invest. 1988;6(1):39–45. doi: 10.3109/07357908809077027. [DOI] [PubMed] [Google Scholar]
- O'Dwyer P. J., Ravikumar T. S., McCabe D. P., Steele G., Jr Effect of 13-cis-retinoic acid on tumor prevention, tumor growth, and metastasis in experimental colon cancer. J Surg Res. 1987 Dec;43(6):550–557. doi: 10.1016/0022-4804(87)90130-2. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Phillips R. W., Kikendall J. W., Luk G. D., Willis S. M., Murphy J. R., Maydonovitch C., Bowen P. E., Stacewicz-Sapuntzakis M., Wong R. K. beta-Carotene inhibits rectal mucosal ornithine decarboxylase activity in colon cancer patients. Cancer Res. 1993 Aug 15;53(16):3723–3725. [PubMed] [Google Scholar]
- Rosewicz S., Stier U., Brembeck F., Kaiser A., Papadimitriou C. A., Berdel W. E., Wiedenmann B., Riecken E. O. Retinoids: effects on growth, differentiation, and nuclear receptor expression in human pancreatic carcinoma cell lines. Gastroenterology. 1995 Nov;109(5):1646–1660. doi: 10.1016/0016-5085(95)90655-x. [DOI] [PubMed] [Google Scholar]
- Silverman J., Katayama S., Zelenakas K., Lauber J., Musser T. K., Reddy M., Levenstein M. J., Weisburger H. Effect of retinoids on the induction of colon cancer in F344 rats by N-methyl-N-nitrosourea or by 1,2-dimethylhydrazine. Carcinogenesis. 1981;2(11):1167–1172. doi: 10.1093/carcin/2.11.1167. [DOI] [PubMed] [Google Scholar]
- Stopera S. A., Bird R. P. Effects of all-trans retinoic acid as a potential chemopreventive agent on the formation of azoxymethane-induced aberrant crypt foci: differential expression of c-myc and c-fos MRNA and protein. Int J Cancer. 1993 Mar 12;53(5):798–803. doi: 10.1002/ijc.2910530516. [DOI] [PubMed] [Google Scholar]
- Tallman M. S., Wiernik P. H. Retinoids in cancer treatment. J Clin Pharmacol. 1992 Oct;32(10):868–888. doi: 10.1002/j.1552-4604.1992.tb04633.x. [DOI] [PubMed] [Google Scholar]
- Ward J. M., Sporn M. B., Wenk M. L., Smith J. M., Feeser D., Dean R. J. Dose response to intrarectal administration of N-methyl-N-nitrosourea and histopathologic evaluation of the effect of two retinoids on colon lesions induced in rats. J Natl Cancer Inst. 1978 Jun;60(6):1489–1493. doi: 10.1093/jnci/60.6.1489. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Liu Y., Lee M. O., Pfahl M. A specific defect in the retinoic acid response associated with human lung cancer cell lines. Cancer Res. 1994 Nov 1;54(21):5663–5669. [PubMed] [Google Scholar]
- de Thé H., Vivanco-Ruiz M. M., Tiollais P., Stunnenberg H., Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990 Jan 11;343(6254):177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]
- van der Leede B. J., Folkers G. E., van den Brink C. E., van der Saag P. T., van der Burg B. Retinoic acid receptor alpha 1 isoform is induced by estradiol and confers retinoic acid sensitivity in human breast cancer cells. Mol Cell Endocrinol. 1995 Mar;109(1):77–86. doi: 10.1016/0303-7207(95)03487-r. [DOI] [PubMed] [Google Scholar]