Abstract
BACKGROUND—Diarrhoea in AIDS is associated with anorexia and weight loss. The importance of gastrointestinal transit in such symptoms has not been addressed. AIMS—To assess jejunal to caecal transit times in subjects with AIDS related diarrhoea and weight loss and correlate these with measures of absorptive capacity and intestinal permeability. METHODS—Jejunal to caecal transit times were assessed in 20 seronegative controls and 60 HIV seropositive subjects from serum analysis of 3-O-methyl-D-glucose and sulphapyridine after ingestion of the monosaccharide and sulphasalazine in aqueous solution. The method also allows an estimation of gastric emptying times for liquids. Intestinal absorptive capacity and permeability were assessed by a combined test using 3-O-methyl-D-glucose, D-xylose, L-rhamnose, and lactulose. RESULTS—Gastric emptying was significantly delayed in all groups of patients with AIDS. Mean jejunal to caecal transit times were not significantly different between controls (246 (62) minutes) and patients without diarrhoea (AIDS, well: 278 (103) minutes; AIDS, wasting: 236 (68) minutes), cytomegalovirus colitis (289 (83) minutes), pathogen negative diarrhoea (192 (100) minutes), or microsporidiosis (190 (113) minutes), although 30% of patients had values below the control range. Patients with cryptosporidiosis differed significantly from controls (135 (35) minutes, p<0.0001), seven of 10 having rapid transit times. Absorptive capacity was reduced and intestinal permeability significantly increased in AIDS, but did not correlate significantly with transit times. CONCLUSION—Small bowel transit is accelerated in many patients with AIDS, particularily in protozoal diarrhoea, but is not the sole explanation for malabsorption of monosaccharides. Keywords: intestinal infection; intestinal absorption; intestinal transit; intestinal function; AIDS; HIV
Full Text
The Full Text of this article is available as a PDF (145.3 KB).
Figure 1 .
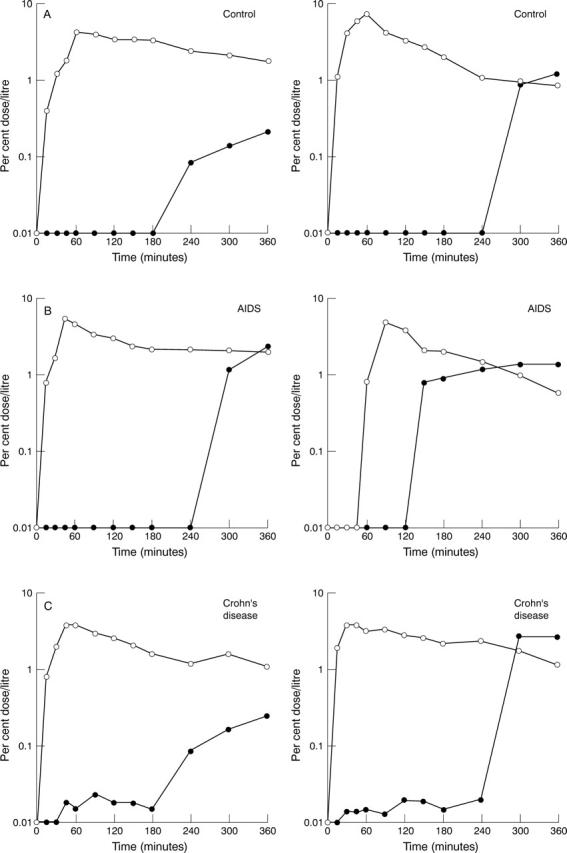
Representative permeation profiles of 3-O-methyl-D-glucose (open circles) and sulphapyridine (closed circles) after ingestion of the monosaccharide and sulphasalazine in two control subjects (A), two patients with AIDS (B), and two patients with Crohn's disease associated with small bowel bacterial overgrowth (C).
Figure 2 .
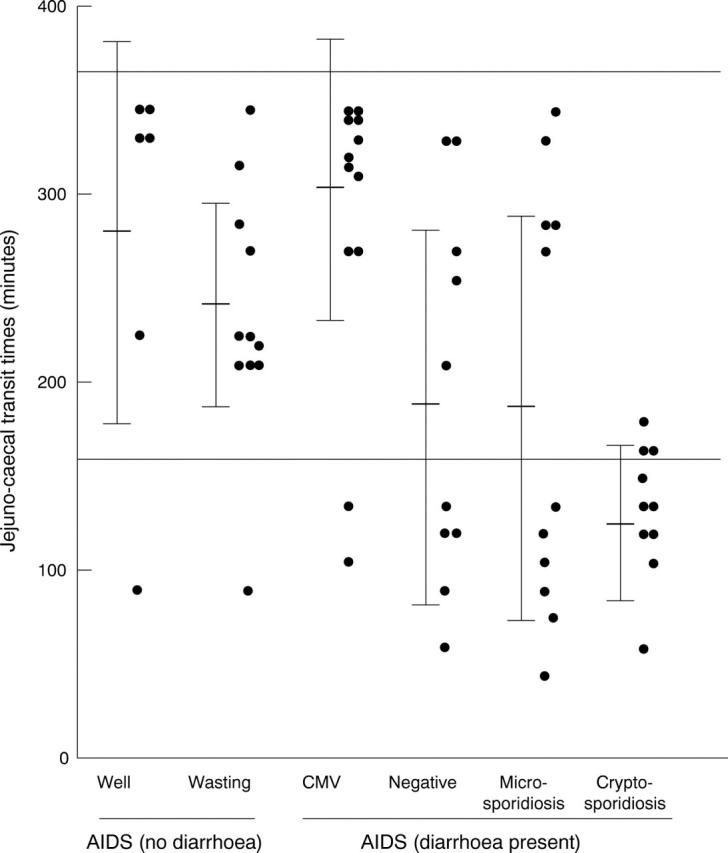
Individual jejunal to caecal transit times in the patients with AIDS. The two horizontal lines represent the upper and lower normal range as obtained from the 20 control subjects. Horizontal bars represent mean values and the vertical bars represent SD.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batman P. A., Miller A. R., Sedgwick P. M., Griffin G. E. Autonomic denervation in jejunal mucosa of homosexual men infected with HIV. AIDS. 1991 Oct;5(10):1247–1252. doi: 10.1097/00002030-199110000-00015. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., MacPherson A., Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995 May;108(5):1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Sharpstone D. R., Francis N., Marker A., Taylor C., Barrett M., Macpherson A., Baldwin C., Menzies I. S., Crane R. C. Intestinal inflammation, ileal structure and function in HIV. AIDS. 1996 Oct;10(12):1385–1391. doi: 10.1097/00002030-199610000-00011. [DOI] [PubMed] [Google Scholar]
- Blanshard C., Ellis D. S., Tovey G., Gazzard B. G. Electron microscopy of rectal biopsies in HIV-positive individuals. J Pathol. 1993 Jan;169(1):79–87. doi: 10.1002/path.1711690113. [DOI] [PubMed] [Google Scholar]
- Budhraja M., Levendoglu H., Kocka F., Mangkornkanok M., Sherer R. Duodenal mucosal T cell subpopulation and bacterial cultures in acquired immune deficiency syndrome. Am J Gastroenterol. 1987 May;82(5):427–431. [PubMed] [Google Scholar]
- Chungi V. S., Rekhi G. S., Shargel L. A simple and rapid liquid chromatographic method for the determination of major metabolites of sulfasalazine in biological fluids. J Pharm Sci. 1989 Mar;78(3):235–238. doi: 10.1002/jps.2600780313. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Dawson V. L. gp120 neurotoxicity in primary cortical cultures. Adv Neuroimmunol. 1994;4(3):167–173. doi: 10.1016/s0960-5428(06)80253-6. [DOI] [PubMed] [Google Scholar]
- FORDTRAN J. S., CLODI P. H., SOERGEL K. H., INGELFINGER F. J. Sugar absorption tests, with special reference to 3-0-methyl-d-glucose and d-xylose. Ann Intern Med. 1962 Dec;57:883–891. doi: 10.7326/0003-4819-57-6-883. [DOI] [PubMed] [Google Scholar]
- Gerding D. N. Diagnosis of Clostridium difficile--associated disease: patient selection and test perfection. Am J Med. 1996 May;100(5):485–486. doi: 10.1016/s0002-9343(95)00057-7. [DOI] [PubMed] [Google Scholar]
- Grohmann G. S., Glass R. I., Pereira H. G., Monroe S. S., Hightower A. W., Weber R., Bryan R. T. Enteric viruses and diarrhea in HIV-infected patients. Enteric Opportunistic Infections Working Group. N Engl J Med. 1993 Jul 1;329(1):14–20. doi: 10.1056/NEJM199307013290103. [DOI] [PubMed] [Google Scholar]
- Hongo M., Okuno Y. Diabetic gastropathy in patients with autonomic neuropathy. Diabet Med. 1993;10 (Suppl 2):79S–81S. doi: 10.1111/j.1464-5491.1993.tb00207.x. [DOI] [PubMed] [Google Scholar]
- Katz D. A., Lynch M. E., Littenberg B. Clinical prediction rules to optimize cytotoxin testing for Clostridium difficile in hospitalized patients with diarrhea. Am J Med. 1996 May;100(5):487–495. doi: 10.1016/s0002-9343(95)00016-x. [DOI] [PubMed] [Google Scholar]
- Keating J., Bjarnason I., Somasundaram S., Macpherson A., Francis N., Price A. B., Sharpstone D., Smithson J., Menzies I. S., Gazzard B. G. Intestinal absorptive capacity, intestinal permeability and jejunal histology in HIV and their relation to diarrhoea. Gut. 1995 Nov;37(5):623–629. doi: 10.1136/gut.37.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellow J. E., Borody T. J., Phillips S. F., Haddad A. C., Brown M. L. Sulfapyridine appearance in plasma after salicylazosulfapyridine. Another simple measure of intestinal transit. Gastroenterology. 1986 Aug;91(2):396–400. doi: 10.1016/0016-5085(86)90574-3. [DOI] [PubMed] [Google Scholar]
- Kennedy M., Chinwah P., Wade D. N. A pharmacological method of measuring mouth caecal transit time in man. Br J Clin Pharmacol. 1979 Oct;8(4):372–373. doi: 10.1111/j.1365-2125.1979.tb04723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch G. T., Thea D. M. Malnutrition in AIDS. Med Clin North Am. 1993 Jul;77(4):795–814. doi: 10.1016/s0025-7125(16)30225-5. [DOI] [PubMed] [Google Scholar]
- Kotler D. P., Reka S., Clayton F. Intestinal mucosal inflammation associated with human immunodeficiency virus infection. Dig Dis Sci. 1993 Jun;38(6):1119–1127. doi: 10.1007/BF01295730. [DOI] [PubMed] [Google Scholar]
- Lim S. G., Menzies I. S., Lee C. A., Johnson M. A., Pounder R. E. Intestinal permeability and function in patients infected with human immunodeficiency virus. A comparison with coeliac disease. Scand J Gastroenterol. 1993 Jul;28(7):573–580. doi: 10.3109/00365529309096090. [DOI] [PubMed] [Google Scholar]
- Macallan D. C., Noble C., Baldwin C., Jebb S. A., Prentice A. M., Coward W. A., Sawyer M. B., McManus T. J., Griffin G. E. Energy expenditure and wasting in human immunodeficiency virus infection. N Engl J Med. 1995 Jul 13;333(2):83–88. doi: 10.1056/NEJM199507133330202. [DOI] [PubMed] [Google Scholar]
- Menzies I. S., Mount J. N., Wheeler M. J. Quantitative estimation of clinically important monosaccharides in plasma by rapid thin layer chromatography. Ann Clin Biochem. 1978 Mar;15(2):65–76. doi: 10.1177/000456327801500116. [DOI] [PubMed] [Google Scholar]
- Noone C., Menzies I. S., Banatvala J. E., Scopes J. W. Intestinal permeability and lactose hydrolysis in human rotaviral gastroenteritis assessed simultaneously by non-invasive differential sugar permeation. Eur J Clin Invest. 1986 Jun;16(3):217–225. doi: 10.1111/j.1365-2362.1986.tb01332.x. [DOI] [PubMed] [Google Scholar]
- Ott M., Lembcke B., Staszewski S., Helm E. B., Caspary W. F. Intestinale Permeabilität bei Patienten mit erworbenem Immundefekt-syndrom (AIDS). Klin Wochenschr. 1991 Oct 2;69(15):715–721. doi: 10.1007/BF01649441. [DOI] [PubMed] [Google Scholar]
- Peppercorn M. A., Goldman P. The role of intestinal bacteria in the metabolism of salicylazosulfapyridine. J Pharmacol Exp Ther. 1972 Jun;181(3):555–562. [PubMed] [Google Scholar]
- Rigaud D., Bedig G., Merrouche M., Vulpillat M., Bonfils S., Apfelbaum M. Delayed gastric emptying in anorexia nervosa is improved by completion of a renutrition program. Dig Dis Sci. 1988 Aug;33(8):919–925. doi: 10.1007/BF01535985. [DOI] [PubMed] [Google Scholar]
- Rüttimann S., Hilti P., Spinas G. A., Dubach U. C. High frequency of human immunodeficiency virus-associated autonomic neuropathy and more severe involvement in advanced stages of human immunodeficiency virus disease. Arch Intern Med. 1991 Dec;151(12):2441–2443. doi: 10.1001/archinte.1991.00400120079013. [DOI] [PubMed] [Google Scholar]
- Sharpstone D. R., Ross H. M., Gazzard B. G. The metabolic response to opportunistic infections in AIDS. AIDS. 1996 Nov;10(13):1529–1533. doi: 10.1097/00002030-199611000-00011. [DOI] [PubMed] [Google Scholar]
- Sharpstone D. R., Rowbottom A. W., Nelson M. R., Lepper M. W., Gazzard B. G. Faecal tumour necrosis factor-alpha in individuals with HIV-related diarrhoea. AIDS. 1996 Aug;10(9):989–994. doi: 10.1097/00002030-199610090-00009. [DOI] [PubMed] [Google Scholar]
- Sharpstone D., Rowbottom A., Nelson M., Gazzard B. The treatment of microsporidial diarrhoea with thalidomide. AIDS. 1995 Jun;9(6):658–659. doi: 10.1097/00002030-199506000-00025. [DOI] [PubMed] [Google Scholar]
- Teahon K., Somasundaram S., Smith T., Menzies I., Bjarnason I. Assessing the site of increased intestinal permeability in coeliac and inflammatory bowel disease. Gut. 1996 Jun;38(6):864–869. doi: 10.1136/gut.38.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber R., Bryan R. T., Owen R. L., Wilcox C. M., Gorelkin L., Visvesvara G. S. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. The Enteric Opportunistic Infections Working Group. N Engl J Med. 1992 Jan 16;326(3):161–166. doi: 10.1056/NEJM199201163260304. [DOI] [PubMed] [Google Scholar]


