Abstract
BACKGROUND—Tumour necrosis factor (TNF) is a predominant cytokine produced in the gastric mucosa of patients with Helicobacter pylori infection. TNF induces apoptosis in a variety of cells. The soluble TNF receptors (sTNF-Rs) can be divided into sTNF-RI and sTNF-RII, both of which inhibit TNF activity. However, their precise mechanisms remain unclear. AIM—To investigate the role of sTNF-Rs in H pylori infection. METHODS—In 40 patients, production of TNF and sTNF-Rs in gastric mucosa was measured using biopsy specimens. In addition, in gastric epithelial cells, sTNF-R release in response to TNF and the protective effect of sTNF-Rs against the cytotoxic and apoptotic activities of TNF were examined. RESULTS—TNF and sTNF-R expression was significantly higher in H pylori positive than H pylori negative patients. TNF dose-dependently induced sTNF-RI release from gastric epithelial cells. sTNF-RII was also released from the cells. TNF decreased cell viability, but the effect was very small. A combination of anti-sTNF-RI and anti-sTNF-RII monoclonal antibodies significantly increased TNF induced cytotoxicity and apoptosis of gastric epithelial cells. CONCLUSIONS—These results show that sTNF-Rs are actively produced in H pylori infected gastric mucosa. sTNF-Rs appear to protect gastric epithelial cells from TNF induced apoptosis in H pylori infection. Keywords: Helicobacter pylori; tumour necrosis factor; soluble TNF receptors; apoptosis; gastric mucosa; stomach
Full Text
The Full Text of this article is available as a PDF (210.4 KB).
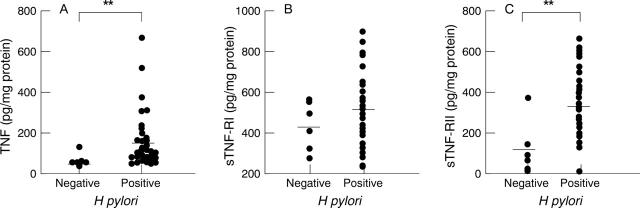
Figure 1 .
Concentrations of tumour necrosis factor (TNF) and soluble TNF receptors (sTNF-Rs) in tissue culture supernatants of antral mucosa from patients with and without H pylori infection. (A) TNF; (B) sTNF-RI; (C) sTNF-RII. Bars represent mean values. TNF and sTNF-RII levels increased significantly in H pylori positive subjects (**p<0.01).
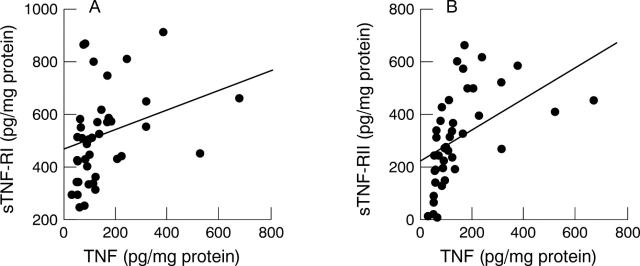
Figure 2 .
Relation between tumour necrosis factor (TNF) and soluble TNF receptor (sTNF-R) concentrations in tissue culture supernatants from H pylori positive subjects. (A) sTNF-RI, p<0.05, r = 0.344; (B) sTNF-RII, p<0.01, r = 0.523.
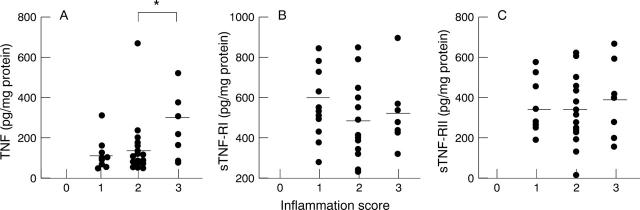
Figure 3 .
Comparison of tumour necrosis factor (TNF) and soluble TNF receptor (sTNF-R) expression according to inflammation score. (A) TNF, *p<0.05; (B) sTNF-RI; (C) sTNF-RII. Bars represent mean values.
Figure 4 .
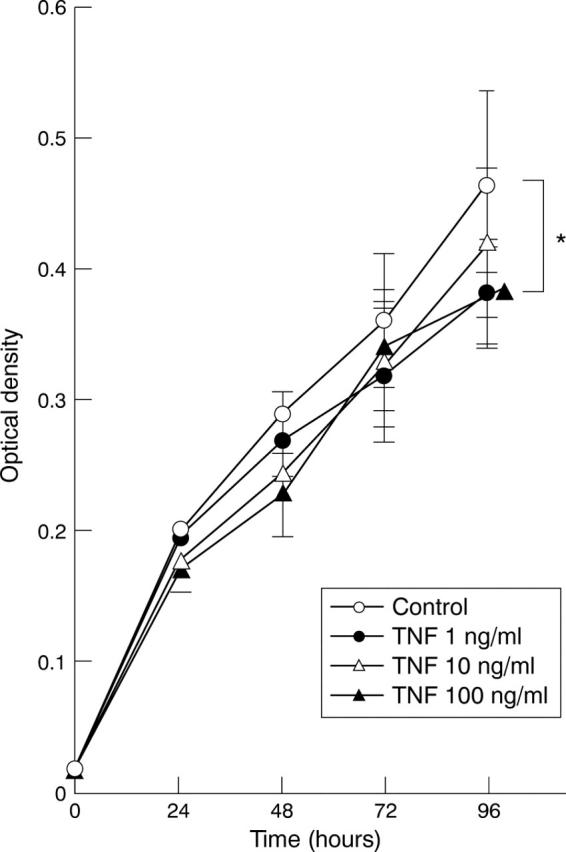
Effect of tumour necrosis factor (TNF) on MKN45 cell viability. Data are expressed as mean (SD) (n = 8). *p<0.05. There are significant differences (p<0.05) between control and 1 ng/ml and 100 ng/ml TNF.
Figure 5 .
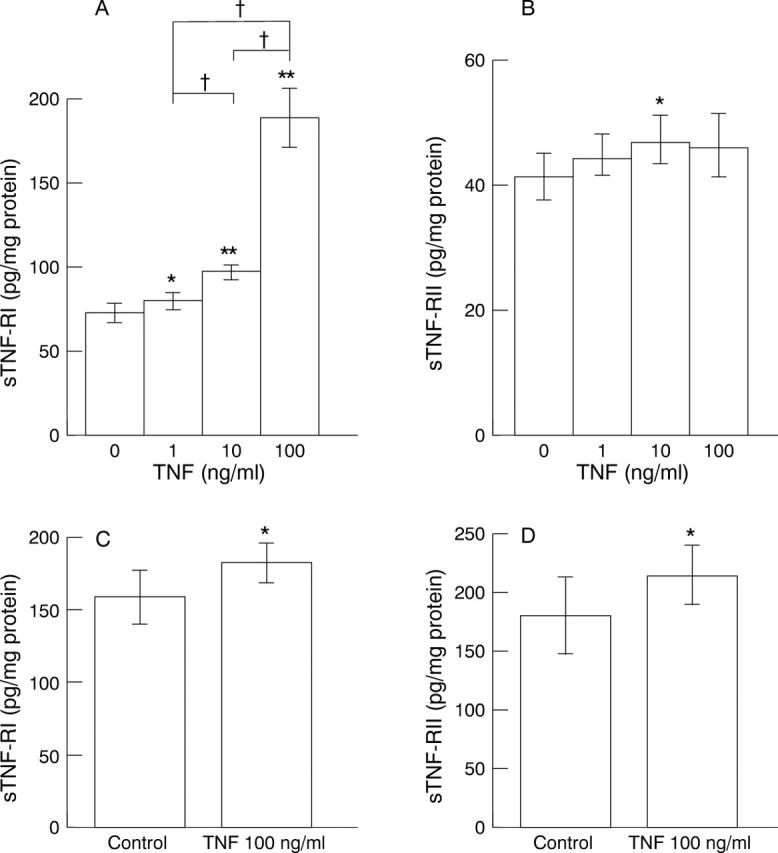
Release of soluble tumour necrosis factor (TNF) receptors (sTNF-Rs) from gastric epithelial cells by various concentrations of TNF for 24 hours. (A) sTNF-RI from MKN45 cells; (B) sTNF-RII from MKN45 cells; (C) sTNF-RI from KATO III cells; (D) sTNF-RII from KATO III cells. Data are expressed as mean (SD) (n = 8). *p<0.05, **p<0.01 v control. †p<0.01.
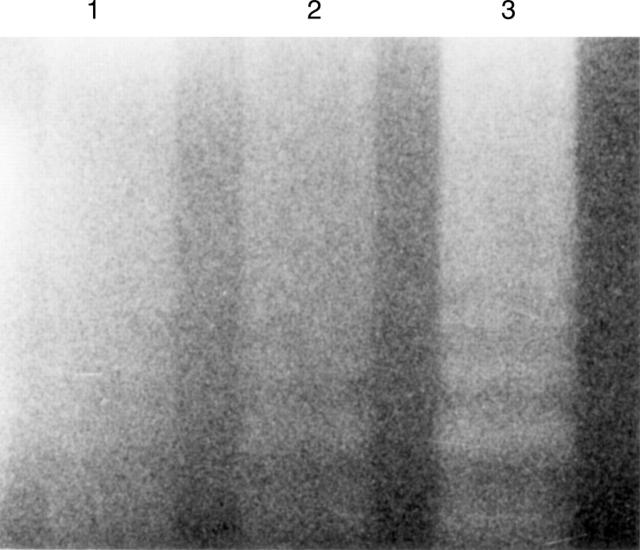
Figure 6 .
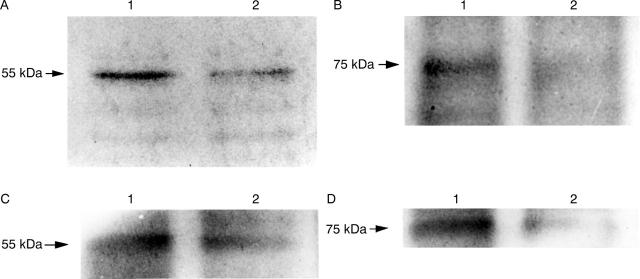
Target of anti-soluble tumour necrosis factor receptor (sTNF-R) monoclonal antibodies. (A) Anti-sTNF-RI; (B) anti-sTNF-RII. Lane 1, MKN45 cell lysate; lane 2, MKN45 cell culture supernatant.
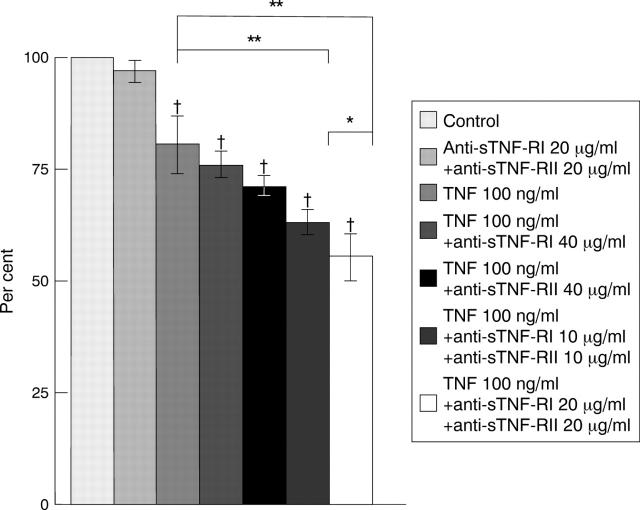
Figure 7 .
Influence of anti-soluble tumour necrosis factor (TNF) receptor I (sTNF-RI) monoclonal antibody and/or anti-sTNF-RII monoclonal antibody on TNF cytotoxicity. The reduction in cell viability was dependent on the concentrations of both types of anti-sTNF-R monoclonal antibody. Data are expressed as mean (SD) (n = 6). †p<0.01 v control. *p<0.05; **p<0.01.
Figure 8 .
Agarose gel electrophoresis of DNA extracted from MKN45 cells exposed to tumour necrosis factor (TNF) and anti-soluble TNF receptor (sTNF-R) monoclonal antibodies for 96 hours. Lane 1, control (no TNF or anti-sTNF-R monoclonal antibodies); lane 2, TNF 100 ng/ml; lane 3, TNF 100 ng/ml + anti-sTNF-RI and anti-sTNF-RII monoclonal antibodies 20 µg/ml. Addition of anti-sTNF-R monoclonal antibodies induced a more conspicuous DNA ladder.
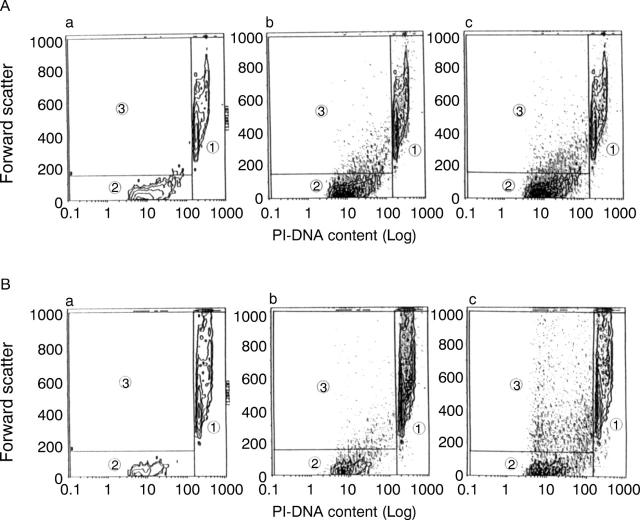
Figure 9 .
Detection of apoptosis by flow cytometric analysis. (A) MKN45 cells. (a) Cells were cultured for 96 hours in control medium; (b) cells were treated with tumour necrosis factor (TNF) 100 ng/ml alone; (c) cells were treated with TNF 100 ng/ml + anti-soluble TNF receptor I (sTNF-RI) and anti-sTNF-RII monoclonal antibodies 20 µg/ml. (B) KATO III cells. (a)-(c) are the same as for MKN45 cells. These are representative of two dimensional frequency contour plots of forward scatter (linear scale) v propidium iodide (PI)-DNA content (logarithmic scale). Apoptosis was recognised in area 3. (A) (a), (b), (c): 3.0%, 6.2%, 9.6% respectively. (B) (a), (b), (c): 3.0%, 5.9%, 20.2% respectively. Addition of anti-sTNF-R monoclonal antibodies induced apoptosis more conspicuously (p<0.01).
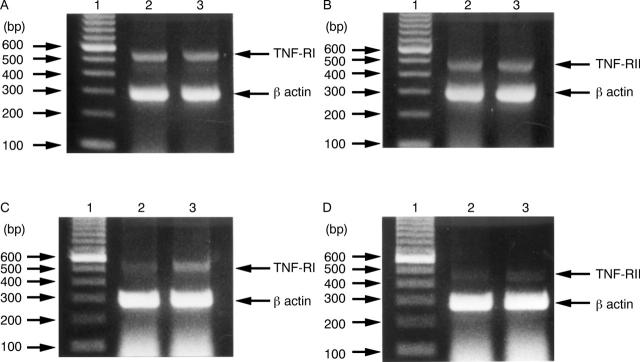
Figure 10 .
Detection of mRNA for tumour necrosis factor receptor I (TNF-RI) and TNF-RII in gastric epithelial cells by reverse transcriptase polymerase chain reaction (PCR). Ethidium bromide stained gels containing PCR products of reverse transcribed TNF-RI mRNA (A) and TNF-RII mRNA (B) in MKN45 cells, and TNF-RI mRNA (C) and TNF-RII mRNA (D) in KATO III cells. Lane 1, 100 bp DNA markers; lane 2, mRNA from untreated cells; lane 3, mRNA from cells treated with TNF 100 ng/ml for 96 hours. In (A) and (B), mRNA of lanes 2 and 3 were expressed equally. In (C) and (D), mRNA of lane 3 was expressed slightly more strongly than that of lane 2.
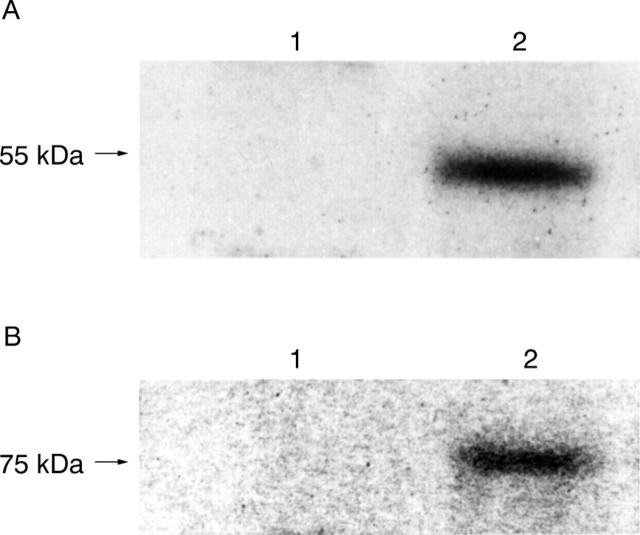
Figure 11 .
Immunoblotting analysis of membrane associated tumour necrosis factor (TNF) receptor I (TNF-RI) and TNF-RII on gastric epithelial cells with and without TNF (100 ng/ml) for 96 hours. (A) 55 kDa TNF-RI in MKN45 cells; (B) 75 kDa TNF-RII in MKN45 cells; (C) 55 kDa TNF-RI in KATO III cells; (D) 75 kDa TNF-RII in KATO III cells. Lane 1, untreated; lane 2, treated with TNF. Expression of TNF-RI and TNF-RII decreased after treatment with TNF.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abreu-Martin M. T., Vidrich A., Lynch D. H., Targan S. R. Divergent induction of apoptosis and IL-8 secretion in HT-29 cells in response to TNF-alpha and ligation of Fas antigen. J Immunol. 1995 Nov 1;155(9):4147–4154. [PubMed] [Google Scholar]
- Austgulen R., Liabakk N. B., Brockhaus M., Espevik T. Soluble TNF receptors in amniotic fluid and in urine from pregnant women. J Reprod Immunol. 1992 Aug;22(2):105–116. doi: 10.1016/0165-0378(92)90009-s. [DOI] [PubMed] [Google Scholar]
- Bader T., Nettesheim P. Tumor necrosis factor-alpha modulates the expression of its p60 receptor and several cytokines in rat tracheal epithelial cells. J Immunol. 1996 Oct 1;157(7):3089–3096. [PubMed] [Google Scholar]
- Crabtree J. E., Shallcross T. M., Heatley R. V., Wyatt J. I. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991 Dec;32(12):1473–1477. doi: 10.1136/gut.32.12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dive C., Gregory C. D., Phipps D. J., Evans D. L., Milner A. E., Wyllie A. H. Analysis and discrimination of necrosis and apoptosis (programmed cell death) by multiparameter flow cytometry. Biochim Biophys Acta. 1992 Feb 3;1133(3):275–285. doi: 10.1016/0167-4889(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Douvdevani A., Einbinder T., Yulzari R., Rogachov B., Chaimovitz C. TNF-receptors on human peritoneal mesothelial cells: regulation of receptor levels and shedding by IL-1 alpha and TNF alpha. Kidney Int. 1996 Jul;50(1):219–228. doi: 10.1038/ki.1996.305. [DOI] [PubMed] [Google Scholar]
- Dummer R., Heald P. W., Nestle F. O., Ludwig E., Laine E., Hemmi S., Burg G. Sézary syndrome T-cell clones display T-helper 2 cytokines and express the accessory factor-1 (interferon-gamma receptor beta-chain). Blood. 1996 Aug 15;88(4):1383–1389. [PubMed] [Google Scholar]
- Fernandez-Botran R. Soluble cytokine receptors: their role in immunoregulation. FASEB J. 1991 Aug;5(11):2567–2574. doi: 10.1096/fasebj.5.11.1868981. [DOI] [PubMed] [Google Scholar]
- Fiorucci S., Santucci L., Migliorati G., Riccardi C., Amorosi A., Mancini A., Roberti R., Morelli A. Isolated guinea pig gastric chief cells express tumour necrosis factor receptors coupled with the sphingomyelin pathway. Gut. 1996 Feb;38(2):182–189. doi: 10.1136/gut.38.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman D., Newell D. G., Fullerton F., Yarnell J. W., Stacey A. R., Wald N., Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991 Jun 1;302(6788):1302–1305. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lloret M. I., Yui J., Winkler-Lowen B., Guilbert L. J. Epidermal growth factor inhibits cytokine-induced apoptosis of primary human trophoblasts. J Cell Physiol. 1996 May;167(2):324–332. doi: 10.1002/(SICI)1097-4652(199605)167:2<324::AID-JCP17>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Girardin E., Roux-Lombard P., Grau G. E., Suter P., Gallati H., Dayer J. M. Imbalance between tumour necrosis factor-alpha and soluble TNF receptor concentrations in severe meningococcaemia. The J5 Study Group. Immunology. 1992 May;76(1):20–23. [PMC free article] [PubMed] [Google Scholar]
- Heaney M. L., Golde D. W. Soluble hormone receptors. Blood. 1993 Oct 1;82(7):1945–1948. [PubMed] [Google Scholar]
- Higuchi M., Aggarwal B. B. Differential roles of two types of the TNF receptor in TNF-induced cytotoxicity, DNA fragmentation, and differentiation. J Immunol. 1994 Apr 15;152(8):4017–4025. [PubMed] [Google Scholar]
- Ishizuka T., Morita K., Hisada T., Ishizuka T., Ando S., Adachi M., Dobashi K., Mori M. The direct effect of interferon-gamma on human eosinophilic leukemia cell lines: the induction of interleukin-5 mRNA and the presence of an interferon-gamma receptor. Inflammation. 1996 Apr;20(2):151–163. doi: 10.1007/BF01487402. [DOI] [PubMed] [Google Scholar]
- Kalinkovich A., Engelmann H., Harpaz N., Burstein R., Barak V., Kalickman I., Wallach D., Bentwich Z. Elevated serum levels of soluble tumour necrosis factor receptors (sTNF-R) in patients with HIV infection. Clin Exp Immunol. 1992 Sep;89(3):351–355. doi: 10.1111/j.1365-2249.1992.tb06961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno T., Brewer M. T., Baker S. L., Schwartz P. E., King M. W., Hale K. K., Squires C. H., Thompson R. C., Vannice J. L. A second tumor necrosis factor receptor gene product can shed a naturally occurring tumor necrosis factor inhibitor. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8331–8335. doi: 10.1073/pnas.87.21.8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. T., Wang L. Y., Wang J. T., Wang T. H., Yang C. S., Chen C. J. A nested case-control study on the association between Helicobacter pylori infection and gastric cancer risk in a cohort of 9775 men in Taiwan. Anticancer Res. 1995 Mar-Apr;15(2):603–606. [PubMed] [Google Scholar]
- Mannick E. E., Bravo L. E., Zarama G., Realpe J. L., Zhang X. J., Ruiz B., Fontham E. T., Mera R., Miller M. J., Correa P. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 1996 Jul 15;56(14):3238–3243. [PubMed] [Google Scholar]
- Marshall B. J., Warren J. R., Francis G. J., Langton S. R., Goodwin C. S., Blincow E. D. Rapid urease test in the management of Campylobacter pyloridis-associated gastritis. Am J Gastroenterol. 1987 Mar;82(3):200–210. [PubMed] [Google Scholar]
- Moss S. F., Calam J., Agarwal B., Wang S., Holt P. R. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996 Apr;38(4):498–501. doi: 10.1136/gut.38.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M., Pike-Nobile L., Soo V. W., Epstein L. B. Characterization of TNF receptors on human thymocytes. Thymus. 1994;23(3-4):177–194. [PubMed] [Google Scholar]
- Nicoletti I., Migliorati G., Pagliacci M. C., Grignani F., Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991 Jun 3;139(2):271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- Nielsen H., Andersen L. P. Chemotactic activity of Helicobacter pylori sonicate for human polymorphonuclear leucocytes and monocytes. Gut. 1992 Jun;33(6):738–742. doi: 10.1136/gut.33.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noach L. A., Bosma N. B., Jansen J., Hoek F. J., van Deventer S. J., Tytgat G. N. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994 May;29(5):425–429. doi: 10.3109/00365529409096833. [DOI] [PubMed] [Google Scholar]
- Noguchi K., Naito M., Kataoka S., Yonehara S., Tsuruo T. A recessive mutant of the U937 cell line acquired resistance to anti-Fas and anti-p55 tumor necrosis factor receptor antibody-induced apoptosis. Cell Growth Differ. 1995 Oct;6(10):1271–1277. [PubMed] [Google Scholar]
- Nomura A., Stemmermann G. N., Chyou P. H., Kato I., Perez-Perez G. I., Blaser M. J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991 Oct 17;325(16):1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Price A. B. The Sydney System: histological division. J Gastroenterol Hepatol. 1991 May-Jun;6(3):209–222. doi: 10.1111/j.1440-1746.1991.tb01468.x. [DOI] [PubMed] [Google Scholar]
- Tartaglia L. A., Goeddel D. V. Two TNF receptors. Immunol Today. 1992 May;13(5):151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- Van Zee K. J., Kohno T., Fischer E., Rock C. S., Moldawer L. L., Lowry S. F. Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor alpha in vitro and in vivo. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4845–4849. doi: 10.1073/pnas.89.11.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermes I., Haanen C., Steffens-Nakken H., Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995 Jul 17;184(1):39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- Wagner S., Beil W., Westermann J., Logan R. P., Bock C. T., Trautwein C., Bleck J. S., Manns M. P. Regulation of gastric epithelial cell growth by Helicobacter pylori: offdence for a major role of apoptosis. Gastroenterology. 1997 Dec;113(6):1836–1847. doi: 10.1016/s0016-5085(97)70003-9. [DOI] [PubMed] [Google Scholar]
- Yano N., Endoh M., Naka R., Takemura F., Nomoto Y., Sakai H. Altered synthesis of interferon-gamma and expression of interferon-gamma receptor by peripheral blood mononuclear cells from patients with IgA nephropathy and non-IgA proliferative glomerulonephritis. J Clin Immunol. 1996 Jan;16(1):71–79. doi: 10.1007/BF01540975. [DOI] [PubMed] [Google Scholar]
- van der Poll T., Coyle S. M., Kumar A., Barbosa K., Agosti J. M., Lowry S. F. Down-regulation of surface receptors for TNF and IL-1 on circulating monocytes and granulocytes during human endotoxemia: effect of neutralization of endotoxin-induced TNF activity by infusion of a recombinant dimeric TNF receptor. J Immunol. 1997 Feb 1;158(3):1490–1497. [PubMed] [Google Scholar]