Abstract
BACKGROUND—Although motor and sensory pathways to the human external anal sphincter are bilateral, a unilateral pudendal neuropathy may still disrupt anal continence. Anal continence can, however, be preserved despite unilateral pudendal damage, and so to explain those differing observations, we postulated that pudendal innervation might be asymmetric. AIMS—To explore the individual effects of right and left pudendal nerve stimulation on the corticofugal pathways to the human external anal sphincter and thus assess evidence for functional asymmetric pelvic innervation. METHODS—In eight healthy subjects, anal sphincter electromyographic responses, evoked to transcranial magnetic stimulation of the motor cortex, were recorded 5-500 msec after digital transrectal electrical conditioning stimuli applied to each pudendal nerve. RESULTS—Right or left pudendal nerve stimulation evoked anal responses of similar latencies but asymmetric amplitudes in six subjects: dominant responses (>50% contralateral side) from the right pudendal in four subjects and from the left in two. Cortical stimulation also evoked anal responses with amplitude 448 (121) µV and latency 20.9 (1.1) msec. When cortical stimulation was preceded by pudendal nerve stimulation, the cortical responses were facilitated at interstimulus intervals of 5-20 msec. Dominant pudendal nerve stimulation induced greater facilitation of the cortically evoked responses than the non-dominant nerve. CONCLUSIONS—Cortical pathways to the external anal sphincter are facilitated by pudendal nerve conditioning, in an asymmetric manner. This functional asymmetry may explain the presence and absence of anal incontinence after unilateral pudendal nerve injury. Keywords: cerebral cortex; continence; electromyography; external anal sphincter; incontinence; magnetic stimulation
Full Text
The Full Text of this article is available as a PDF (127.0 KB).
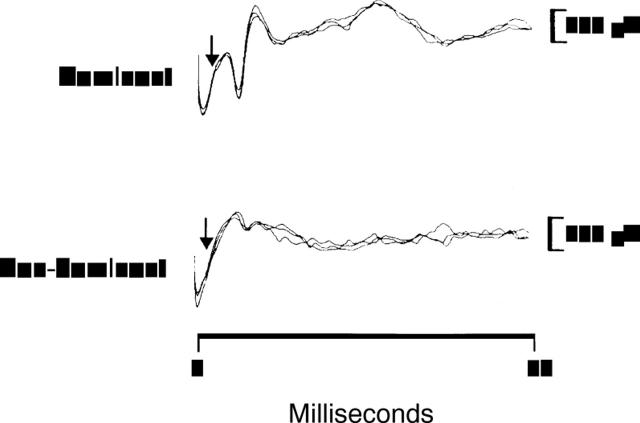
Figure 1 .
External anal sphincter EMG responses are shown following right (dominant) and left (non-dominant) pudendal nerve stimulation in one individual. Three traces are superimposed to show reproducibility. Stimulus at 0 ms; arrows indicate the onset of the EMG response. The response amplitude from the dominant pudendal nerve is larger than from the non-dominant nerve, despite similar latencies.
Figure 2 .
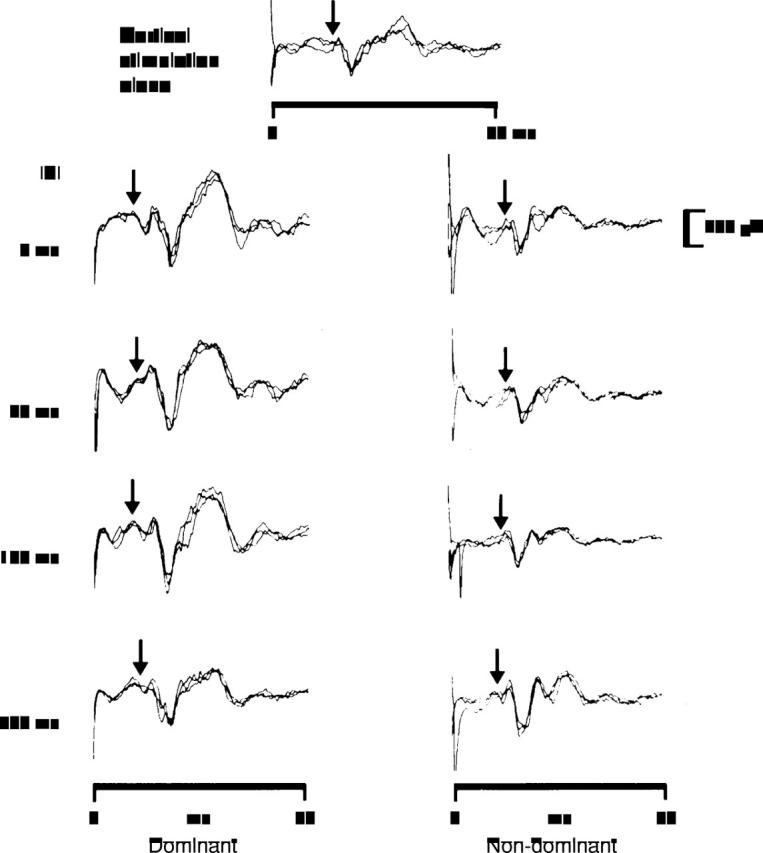
Cortically evoked external anal sphincter EMG responses from one individual following cortical stimulation alone and following dominant and non-dominant pudendal nerve stimulation at different ISIs. Three traces are superimposed to show reproducibility. Cortical stimulus at 0 msec; arrows indicate the onset of the EMG response. Following dominant pudendal nerve stimulation, the cortically evoked responses are facilitated to a much greater degree than with non-dominant pudendal stimulation.
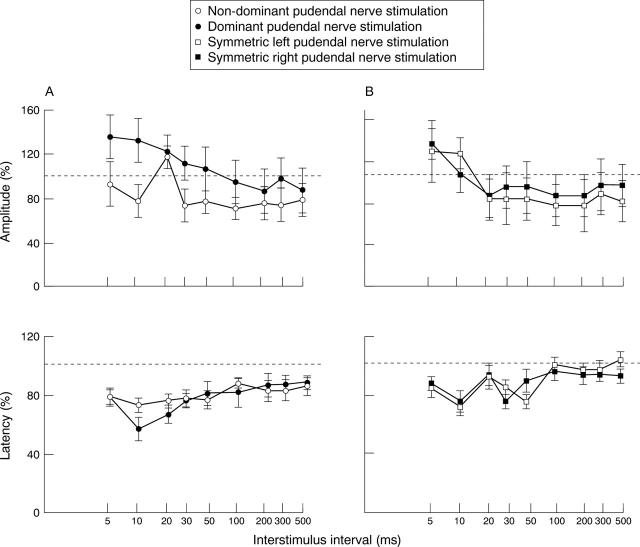
Figure 3 .
Effects of: (A) dominant (closed circle) and non-dominant pudendal nerve (open circle) stimulation (n=6); and (B) symmetric right (closed square) and left (open square) pudendal nerve stimulation (n=2) on the amplitudes and latencies of the cortically evoked anal responses at increasing ISIs. The vertical axes show the percentage of the response to cortical stimulation alone; the horizontal broken line indicates 100%. Dominant pudendal nerve stimulation produces relatively greater facilitation of the cortically evoked responses than non-dominant pudendal nerve stimulation (p<0.001); in contrast, prior symmetrical pudendal nerve stimulation has equal effects from either side.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP B., GARRY R. C., ROBERTS T. D., TODD J. K. Control of the external sphincter of the anus in the cat. J Physiol. 1956 Oct 29;134(1):229–240. doi: 10.1113/jphysiol.1956.sp005639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst J. C., Andrews K., Richards B., Laycock P. J. Incidence and correlates of incontinence in stroke patients. J Am Geriatr Soc. 1985 Aug;33(8):540–542. doi: 10.1111/j.1532-5415.1985.tb04618.x. [DOI] [PubMed] [Google Scholar]
- Deletis V., Vodusek D. B., Abbott R., Epstein F. J., Turndorf H. Intraoperative monitoring of the dorsal sacral roots: minimizing the risk of iatrogenic micturition disorders. Neurosurgery. 1992 Jan;30(1):72–75. doi: 10.1227/00006123-199201000-00013. [DOI] [PubMed] [Google Scholar]
- Hamdy S., Enck P., Aziz Q., Rothwell J. C., Uengoergil S., Hobson A., Thompson D. G. Spinal and pudendal nerve modulation of human corticoanal motor pathways. Am J Physiol. 1998 Feb;274(2 Pt 1):G419–G423. doi: 10.1152/ajpgi.1998.274.2.G419. [DOI] [PubMed] [Google Scholar]
- Herdmann J., Enck P., Zacchi-Deutschbein P., Ostermann U. Speed and pressure characteristics of external anal sphincter contractions. Am J Physiol. 1995 Aug;269(2 Pt 1):G225–G231. doi: 10.1152/ajpgi.1995.269.2.G225. [DOI] [PubMed] [Google Scholar]
- Hinds J. P., Eidelman B. H., Wald A. Prevalence of bowel dysfunction in multiple sclerosis. A population survey. Gastroenterology. 1990 Jun;98(6):1538–1542. doi: 10.1016/0016-5085(90)91087-m. [DOI] [PubMed] [Google Scholar]
- Kafka N. J., Coller J. A., Barrett R. C., Murray J. J., Roberts P. L., Rusin L. C., Schoetz D. J., Jr Pudendal neuropathy is the only parameter differentiating leakage from solid stool incontinence. Dis Colon Rectum. 1997 Oct;40(10):1220–1227. doi: 10.1007/BF02055168. [DOI] [PubMed] [Google Scholar]
- Kiff E. S., Swash M. Normal proximal and delayed distal conduction in the pudendal nerves of patients with idiopathic (neurogenic) faecal incontinence. J Neurol Neurosurg Psychiatry. 1984 Aug;47(8):820–823. doi: 10.1136/jnnp.47.8.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiff E. S., Swash M. Slowed conduction in the pudendal nerves in idiopathic (neurogenic) faecal incontinence. Br J Surg. 1984 Aug;71(8):614–616. doi: 10.1002/bjs.1800710817. [DOI] [PubMed] [Google Scholar]
- Kubben F. J., Peeters-Haesevoets A., Engels L. G., Baeten C. G., Schutte B., Arends J. W., Stockbrügger R. W., Blijham G. H. Proliferating cell nuclear antigen (PCNA): a new marker to study human colonic cell proliferation. Gut. 1994 Apr;35(4):530–535. doi: 10.1136/gut.35.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzel K. E., Schmidt R. A., Tanagho E. A. Neuroanatomy of the striated muscular anal continence mechanism. Implications for the use of neurostimulation. Dis Colon Rectum. 1990 Aug;33(8):666–673. doi: 10.1007/BF02150742. [DOI] [PubMed] [Google Scholar]
- McKenna K. E., Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986 Jun 22;248(4):532–549. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- Morita T., Kizu N., Kondo S., Dohkita S., Tsuchida S. Ipsilaterality of motor innervation of canine urethral sphincter. Urol Int. 1988;43(3):149–156. doi: 10.1159/000281328. [DOI] [PubMed] [Google Scholar]
- Rieger N. A., Sarre R. G., Saccone G. T., Schloithe A. C., Wattchow D. A. Correlation of pudendal nerve terminal motor latency with the results of anal manometry. Int J Colorectal Dis. 1997;12(5):303–307. doi: 10.1007/s003840050111. [DOI] [PubMed] [Google Scholar]
- Sangwan Y. P., Coller J. A., Barrett M. S., Murray J. J., Roberts P. L., Schoetz D. J., Jr Unilateral pudendal neuropathy. Significance and implications. Dis Colon Rectum. 1996 Mar;39(3):249–251. doi: 10.1007/BF02049459. [DOI] [PubMed] [Google Scholar]
- Sangwan Y. P., Coller J. A., Barrett R. C., Roberts P. L., Murray J. J., Rusin L., Schoetz D. J., Jr Unilateral pudendal neuropathy. Impact on outcome of anal sphincter repair. Dis Colon Rectum. 1996 Jun;39(6):686–689. doi: 10.1007/BF02056951. [DOI] [PubMed] [Google Scholar]
- Snooks S. J., Barnes P. R., Swash M. Damage to the innervation of the voluntary anal and periurethral sphincter musculature in incontinence: an electrophysiological study. J Neurol Neurosurg Psychiatry. 1984 Dec;47(12):1269–1273. doi: 10.1136/jnnp.47.12.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snooks S. J., Setchell M., Swash M., Henry M. M. Injury to innervation of pelvic floor sphincter musculature in childbirth. Lancet. 1984 Sep 8;2(8402):546–550. doi: 10.1016/s0140-6736(84)90766-9. [DOI] [PubMed] [Google Scholar]
- Sultan A. H., Kamm M. A., Hudson C. N., Thomas J. M., Bartram C. I. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993 Dec 23;329(26):1905–1911. doi: 10.1056/NEJM199312233292601. [DOI] [PubMed] [Google Scholar]
- Tetzschner T., Sørensen M., Jønsson L., Lose G., Christiansen J. Delivery and pudendal nerve function. Acta Obstet Gynecol Scand. 1997 Apr;76(4):324–331. doi: 10.1111/j.1600-0412.1997.tb07986.x. [DOI] [PubMed] [Google Scholar]
- Tetzschner T., Sørensen M., Rasmussen O. O., Lose G., Christiansen J. Pudendal nerve damage increases the risk of fecal incontinence in women with anal sphincter rupture after childbirth. Acta Obstet Gynecol Scand. 1995 Jul;74(6):434–440. doi: 10.3109/00016349509024405. [DOI] [PubMed] [Google Scholar]
- Thor K. B., Morgan C., Nadelhaft I., Houston M., De Groat W. C. Organization of afferent and efferent pathways in the pudendal nerve of the female cat. J Comp Neurol. 1989 Oct 8;288(2):263–279. doi: 10.1002/cne.902880206. [DOI] [PubMed] [Google Scholar]
- Ueyama T., Arakawa H., Mizuno N. Central distribution of efferent and afferent components of the pudendal nerve in rat. Anat Embryol (Berl) 1987;177(1):37–49. doi: 10.1007/BF00325288. [DOI] [PubMed] [Google Scholar]
- Ueyama T., Mizuno N., Takahashi O., Nomura S., Arakawa H., Matsushima R. Central distribution of efferent and afferent components of the pudendal nerve in macaque monkeys. J Comp Neurol. 1985 Feb 22;232(4):548–556. doi: 10.1002/cne.902320411. [DOI] [PubMed] [Google Scholar]
- Wunderlich M., Swash M. The overlapping innervation of the two sides of the external anal sphincter by the pudendal nerves. J Neurol Sci. 1983 Apr;59(1):97–109. doi: 10.1016/0022-510x(83)90084-9. [DOI] [PubMed] [Google Scholar]