Full Text
The Full Text of this article is available as a PDF (140.5 KB).
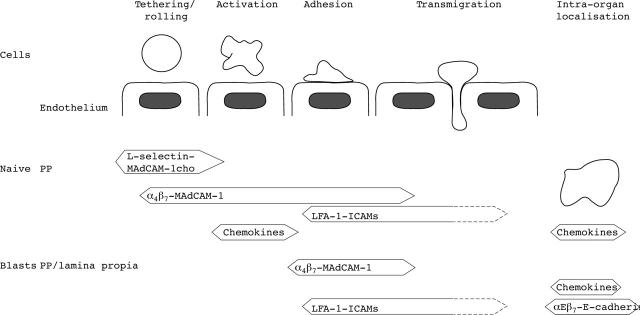
Figure 1 .
Multistep model of lymphocyte homing into the gut. The five separate steps in lymphocyte trafficking from the blood into gut are shown. The molecules mediating different stages of binding of naive lymphocytes to Peyer's patch (PP) high endothelial venules (HEV) and adhesion of activated immunoblasts to Peyer's patch HEV or flat walled venules in lamina propria are shown. In the three first steps only molecules which have been shown to function in vivo under flow conditions in the gut are included. MAdCAM, mucosal addressin cell adhesion molecule; ICAM, intercellular adhesion molecule; LFA, lymphocyte function associated antigen; MAdCAMcho, MAdCAM with special carbohydrate structures.
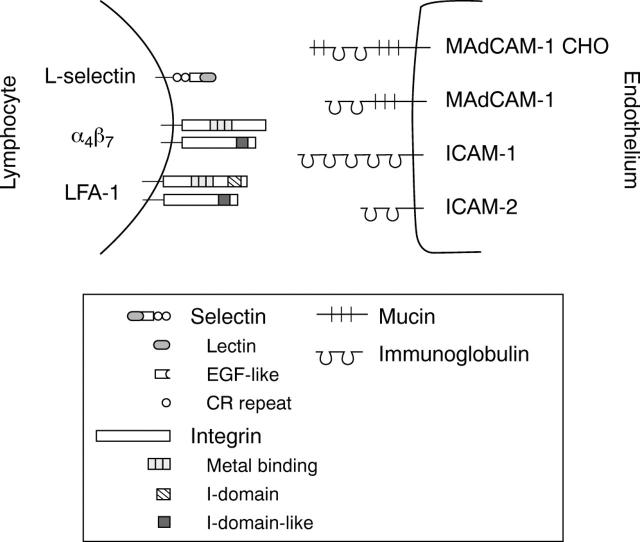
Figure 2 .
Structure of the mucosal adhesion antigens. The mosaic nature of L-selectin, mucosal addressin cell adhesion molecule (MAdCAM) 1 and special carbohydrate containing forms of MAdCAM-1 (MAdCAM-1 CHO) is shown together with the prototypic structure of integrins and intercellular adhesion molecules (ICAM). Lectin, C-type (calcium dependent) lectin domain; EGF-like, epidermal growth factor-like domain; and CR, complement regulatory protein-like motif in selectins. Metal binding = divalent cation binding repeats in α subunits of integrins; I-domain = inserted domain in αL subunit of lymphocyte function associated antigen (LFA) 1 and I-domain-like, a putative inserted domain-like area in β subunits of integrins.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbonés M. L., Ord D. C., Ley K., Ratech H., Maynard-Curry C., Otten G., Capon D. J., Tedder T. F. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994 Jul;1(4):247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Bargatze R. F., Jutila M. A., Butcher E. C. Distinct roles of L-selectin and integrins alpha 4 beta 7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ: the multistep model confirmed and refined. Immunity. 1995 Jul;3(1):99–108. doi: 10.1016/1074-7613(95)90162-0. [DOI] [PubMed] [Google Scholar]
- Berg E. L., McEvoy L. M., Berlin C., Bargatze R. F., Butcher E. C. L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature. 1993 Dec 16;366(6456):695–698. doi: 10.1038/366695a0. [DOI] [PubMed] [Google Scholar]
- Berlin C., Bargatze R. F., Campbell J. J., von Andrian U. H., Szabo M. C., Hasslen S. R., Nelson R. D., Berg E. L., Erlandsen S. L., Butcher E. C. alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995 Feb 10;80(3):413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- Bianchi E., Bender J. R., Blasi F., Pardi R. Through and beyond the wall: late steps in leukocyte transendothelial migration. Immunol Today. 1997 Dec;18(12):586–591. doi: 10.1016/s0167-5699(97)01162-6. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Mucosal immunity in the female genital tract. J Reprod Immunol. 1997 Nov 30;36(1-2):23–50. doi: 10.1016/s0165-0378(97)00061-2. [DOI] [PubMed] [Google Scholar]
- Briskin M., Winsor-Hines D., Shyjan A., Cochran N., Bloom S., Wilson J., McEvoy L. M., Butcher E. C., Kassam N., Mackay C. R. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997 Jul;151(1):97–110. [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Butcher E. C., Picker L. J. Lymphocyte homing and homeostasis. Science. 1996 Apr 5;272(5258):60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Harlan J. M. Leukocyte-endothelial adhesion molecules. Blood. 1994 Oct 1;84(7):2068–2101. [PubMed] [Google Scholar]
- Cepek K. L., Shaw S. K., Parker C. M., Russell G. J., Morrow J. S., Rimm D. L., Brenner M. B. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994 Nov 10;372(6502):190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- Cines D. B., Pollak E. S., Buck C. A., Loscalzo J., Zimmerman G. A., McEver R. P., Pober J. S., Wick T. M., Konkle B. A., Schwartz B. S. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998 May 15;91(10):3527–3561. [PubMed] [Google Scholar]
- DeGrendele H. C., Estess P., Picker L. J., Siegelman M. H. CD44 and its ligand hyaluronate mediate rolling under physiologic flow: a novel lymphocyte-endothelial cell primary adhesion pathway. J Exp Med. 1996 Mar 1;183(3):1119–1130. doi: 10.1084/jem.183.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M. S., Springer T. A. The dynamic regulation of integrin adhesiveness. Curr Biol. 1994 Jun 1;4(6):506–517. doi: 10.1016/s0960-9822(00)00111-1. [DOI] [PubMed] [Google Scholar]
- Erle D. J., Briskin M. J., Butcher E. C., Garcia-Pardo A., Lazarovits A. I., Tidswell M. Expression and function of the MAdCAM-1 receptor, integrin alpha 4 beta 7, on human leukocytes. J Immunol. 1994 Jul 15;153(2):517–528. [PubMed] [Google Scholar]
- Etzioni A. Adhesion molecules--their role in health and disease. Pediatr Res. 1996 Feb;39(2):191–198. doi: 10.1203/00006450-199602000-00001. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G. Leukocyte adhesion: CD11/CD18 integrins and intercellular adhesion molecules. Curr Opin Cell Biol. 1997 Oct;9(5):643–650. doi: 10.1016/s0955-0674(97)80117-2. [DOI] [PubMed] [Google Scholar]
- Girard J. P., Springer T. A. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol Today. 1995 Sep;16(9):449–457. doi: 10.1016/0167-5699(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Gunn M. D., Tangemann K., Tam C., Cyster J. G., Rosen S. D., Williams L. T. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci U S A. 1998 Jan 6;95(1):258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The gut-associated lymphoid system: nature and properties of the large dividing cells. Eur J Immunol. 1974 Jun;4(6):435–443. doi: 10.1002/eji.1830040610. [DOI] [PubMed] [Google Scholar]
- Hall J. G., Hopkins J., Orlans E. Studies on the lymphocytes of sheep. III. Destination of lymph-borne immunoblasts in relation to their tissue of origin. Eur J Immunol. 1977 Jan;7(1):30–37. doi: 10.1002/eji.1830070108. [DOI] [PubMed] [Google Scholar]
- Hamann A., Andrew D. P., Jablonski-Westrich D., Holzmann B., Butcher E. C. Role of alpha 4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol. 1994 Apr 1;152(7):3282–3293. [PubMed] [Google Scholar]
- Hamann A., Jablonski-Westrich D., Jonas P., Thiele H. G. Homing receptors reexamined: mouse LECAM-1 (MEL-14 antigen) is involved in lymphocyte migration into gut-associated lymphoid tissue. Eur J Immunol. 1991 Dec;21(12):2925–2929. doi: 10.1002/eji.1830211205. [DOI] [PubMed] [Google Scholar]
- Hesterberg P. E., Winsor-Hines D., Briskin M. J., Soler-Ferran D., Merrill C., Mackay C. R., Newman W., Ringler D. J. Rapid resolution of chronic colitis in the cotton-top tamarin with an antibody to a gut-homing integrin alpha 4 beta 7. Gastroenterology. 1996 Nov;111(5):1373–1380. doi: 10.1053/gast.1996.v111.pm8898653. [DOI] [PubMed] [Google Scholar]
- Hänninen A., Jaakkola I., Jalkanen S. Mucosal addressin is required for the development of diabetes in nonobese diabetic mice. J Immunol. 1998 Jun 15;160(12):6018–6025. [PubMed] [Google Scholar]
- Imhof B. A., Dunon D. Leukocyte migration and adhesion. Adv Immunol. 1995;58:345–416. doi: 10.1016/s0065-2776(08)60623-9. [DOI] [PubMed] [Google Scholar]
- Jalkanen S., Bargatze R. F., de los Toyos J., Butcher E. C. Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85-95-kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol. 1987 Aug;105(2):983–990. doi: 10.1083/jcb.105.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansas G. S. Selectins and their ligands: current concepts and controversies. Blood. 1996 Nov 1;88(9):3259–3287. [PubMed] [Google Scholar]
- Kantele A., Kantele J. M., Savilahti E., Westerholm M., Arvilommi H., Lazarovits A., Butcher E. C., Mäkelä P. H. Homing potentials of circulating lymphocytes in humans depend on the site of activation: oral, but not parenteral, typhoid vaccination induces circulating antibody-secreting cells that all bear homing receptors directing them to the gut. J Immunol. 1997 Jan 15;158(2):574–579. [PubMed] [Google Scholar]
- Koopman G., van Kooyk Y., de Graaff M., Meyer C. J., Figdor C. G., Pals S. T. Triggering of the CD44 antigen on T lymphocytes promotes T cell adhesion through the LFA-1 pathway. J Immunol. 1990 Dec 1;145(11):3589–3593. [PubMed] [Google Scholar]
- Kubes P., Jutila M., Payne D. Therapeutic potential of inhibiting leukocyte rolling in ischemia/reperfusion. J Clin Invest. 1995 Jun;95(6):2510–2519. doi: 10.1172/JCI117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurose I., Pothoulakis C., LaMont J. T., Anderson D. C., Paulson J. C., Miyasaka M., Wolf R., Granger D. N. Clostridium difficile toxin A-induced microvascular dysfunction. Role of histamine. J Clin Invest. 1994 Nov;94(5):1919–1926. doi: 10.1172/JCI117542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T. Effector and regulatory lymphoid cells and cytokines in mucosal sites. Curr Top Microbiol Immunol. 1999;236:113–135. doi: 10.1007/978-3-642-59951-4_7. [DOI] [PubMed] [Google Scholar]
- McDermott M. R., Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979 May;122(5):1892–1898. [PubMed] [Google Scholar]
- McEvoy L. M., Sun H., Frelinger J. G., Butcher E. C. Anti-CD43 inhibition of T cell homing. J Exp Med. 1997 Apr 21;185(8):1493–1498. doi: 10.1084/jem.185.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachynski R. K., Wu S. W., Gunn M. D., Erle D. J. Secondary lymphoid-tissue chemokine (SLC) stimulates integrin alpha 4 beta 7-mediated adhesion of lymphocytes to mucosal addressin cell adhesion molecule-1 (MAdCAM-1) under flow. J Immunol. 1998 Jul 15;161(2):952–956. [PubMed] [Google Scholar]
- Panés J., Anderson D. C., Miyasaka M., Granger D. N. Role of leukocyte-endothelial cell adhesion in radiation-induced microvascular dysfunction in rats. Gastroenterology. 1995 Jun;108(6):1761–1769. doi: 10.1016/0016-5085(95)90138-8. [DOI] [PubMed] [Google Scholar]
- Panés J., Granger D. N. Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology. 1998 May;114(5):1066–1090. doi: 10.1016/s0016-5085(98)70328-2. [DOI] [PubMed] [Google Scholar]
- Picarella D., Hurlbut P., Rottman J., Shi X., Butcher E., Ringler D. J. Monoclonal antibodies specific for beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of scid mice reconstituted with CD45RBhigh CD4+ T cells. J Immunol. 1997 Mar 1;158(5):2099–2106. [PubMed] [Google Scholar]
- Podolsky D. K., Lobb R., King N., Benjamin C. D., Pepinsky B., Sehgal P., deBeaumont M. Attenuation of colitis in the cotton-top tamarin by anti-alpha 4 integrin monoclonal antibody. J Clin Invest. 1993 Jul;92(1):372–380. doi: 10.1172/JCI116575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiding-Järbrink M., Nordström I., Granström G., Kilander A., Jertborn M., Butcher E. C., Lazarovits A. I., Holmgren J., Czerkinsky C. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunizations. A molecular basis for the compartmentalization of effector B cell responses. J Clin Invest. 1997 Mar 15;99(6):1281–1286. doi: 10.1172/JCI119286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J. Chemokines. Blood. 1997 Aug 1;90(3):909–928. [PubMed] [Google Scholar]
- Rose M. L., Parrott D. M., Bruce R. G. Migration of lymphoblasts to the small intestine. II. Divergent migration of mesenteric and peripheral immunoblasts to sites of inflammation in the mouse. Cell Immunol. 1976 Nov;27(1):36–46. doi: 10.1016/0008-8749(76)90151-9. [DOI] [PubMed] [Google Scholar]
- Rott L. S., Rosé J. R., Bass D., Williams M. B., Greenberg H. B., Butcher E. C. Expression of mucosal homing receptor alpha4beta7 by circulating CD4+ cells with memory for intestinal rotavirus. J Clin Invest. 1997 Sep 1;100(5):1204–1208. doi: 10.1172/JCI119633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi M., Granfors K., MacDermott R., Jalkanen S. Aberrant binding of lamina propria lymphocytes to vascular endothelium in inflammatory bowel diseases. Gastroenterology. 1994 Mar;106(3):596–605. doi: 10.1016/0016-5085(94)90691-2. [DOI] [PubMed] [Google Scholar]
- Salmi M., Jalkanen S. How do lymphocytes know where to go: current concepts and enigmas of lymphocyte homing. Adv Immunol. 1997;64:139–218. doi: 10.1016/s0065-2776(08)60889-5. [DOI] [PubMed] [Google Scholar]
- Salmi M., Kalimo K., Jalkanen S. Induction and function of vascular adhesion protein-1 at sites of inflammation. J Exp Med. 1993 Dec 1;178(6):2255–2260. doi: 10.1084/jem.178.6.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi M., Rajala P., Jalkanen S. Homing of mucosal leukocytes to joints. Distinct endothelial ligands in synovium mediate leukocyte-subtype specific adhesion. J Clin Invest. 1997 May 1;99(9):2165–2172. doi: 10.1172/JCI119389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall T. J., Bacon K. B. Chemokines, leukocyte trafficking, and inflammation. Curr Opin Immunol. 1994 Dec;6(6):865–873. doi: 10.1016/0952-7915(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Schmits R., Filmus J., Gerwin N., Senaldi G., Kiefer F., Kundig T., Wakeham A., Shahinian A., Catzavelos C., Rak J. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood. 1997 Sep 15;90(6):2217–2233. [PubMed] [Google Scholar]
- Spangrude G. J., Braaten B. A., Daynes R. A. Molecular mechanisms of lymphocyte extravasation. I. Studies of two selective inhibitors of lymphocyte recirculation. J Immunol. 1984 Jan;132(1):354–362. [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Stewart M., Thiel M., Hogg N. Leukocyte integrins. Curr Opin Cell Biol. 1995 Oct;7(5):690–696. doi: 10.1016/0955-0674(95)80111-1. [DOI] [PubMed] [Google Scholar]
- Stockton B. M., Cheng G., Manjunath N., Ardman B., von Andrian U. H. Negative regulation of T cell homing by CD43. Immunity. 1998 Mar;8(3):373–381. doi: 10.1016/s1074-7613(00)80542-7. [DOI] [PubMed] [Google Scholar]
- Streeter P. R., Berg E. L., Rouse B. T., Bargatze R. F., Butcher E. C. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature. 1988 Jan 7;331(6151):41–46. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- Tan K., Casasnovas J. M., Liu J. H., Briskin M. J., Springer T. A., Wang J. H. The structure of immunoglobulin superfamily domains 1 and 2 of MAdCAM-1 reveals novel features important for integrin recognition. Structure. 1998 Jun 15;6(6):793–801. doi: 10.1016/s0969-2126(98)00080-x. [DOI] [PubMed] [Google Scholar]
- Wagner N., Löhler J., Kunkel E. J., Ley K., Leung E., Krissansen G., Rajewsky K., Müller W. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996 Jul 25;382(6589):366–370. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- Warnock R. A., Askari S., Butcher E. C., von Andrian U. H. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J Exp Med. 1998 Jan 19;187(2):205–216. doi: 10.1084/jem.187.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacyshyn B. R., Bowen-Yacyshyn M. B., Jewell L., Tami J. A., Bennett C. F., Kisner D. L., Shanahan W. R., Jr A placebo-controlled trial of ICAM-1 antisense oligonucleotide in the treatment of Crohn's disease. Gastroenterology. 1998 Jun;114(6):1133–1142. doi: 10.1016/s0016-5085(98)70418-4. [DOI] [PubMed] [Google Scholar]