Abstract
BACKGROUND/AIMS—Intestinal fibrosis and stricture formation is an unresolved problem in Crohn's disease. The aim of this study was to investigate whether mast cells accumulate in these tissues and whether their localisation is associated with extracellular matrix components. METHODS—Mast cells were visualised by immunohistochemical staining of the mast cell specific proteases chymase and tryptase. Their localisation in relation to extracellular matrix components was shown by immunohistochemical double labelling. RESULTS—In strictures in Crohn's disease, a striking accumulation of mast cells was seen particularly in the hypertrophied and fibrotic muscularis propria, with a mean (SEM) mast cell number of 81.3 (14.9) v 1.5 (0.9)/mm2 in normal bowel (p<0.0005). All mast cells in the muscularis propria were colocalised with patches of laminin. In contrast, in the submucosa, laminin was exclusively found in the basal lamina of blood vessels where many adherent mast cells were seen. No colocalisation of mast cells was found with fibronectin or vitronectin. CONCLUSIONS—The large accumulation of mast cells in the muscle layer of strictured bowel suggests a functional role for these cells in the hypertrophic and fibrotic response of the smooth muscle cells. The colocalisation with laminin indicates a mechanism of interaction between smooth muscle cells and mast cells that may be important in the role of mast cells in the process of fibrosis. Keywords: mast cells; Crohn's disease; fibrosis; strictures; smooth muscle cells; laminin
Full Text
The Full Text of this article is available as a PDF (226.0 KB).
Figure 1 .

Distribution of mast cells in normal colonic tissue. Mast cells were visualised by immunohistochemical staining for mast cell tryptase (pink colour) with the biotin-avidin-peroxidase system. The mucosa, submucosa, and muscularis propria are shown. Mast cells are found in the mucosa and submucosa. Hardly any mast cells are detectable in the muscularis propria. Of the two mast cells seen in the muscularis propria (arrows), one is located in a connective tissue septum reaching from the submucosa into the muscle layer. Original magnification ×50.
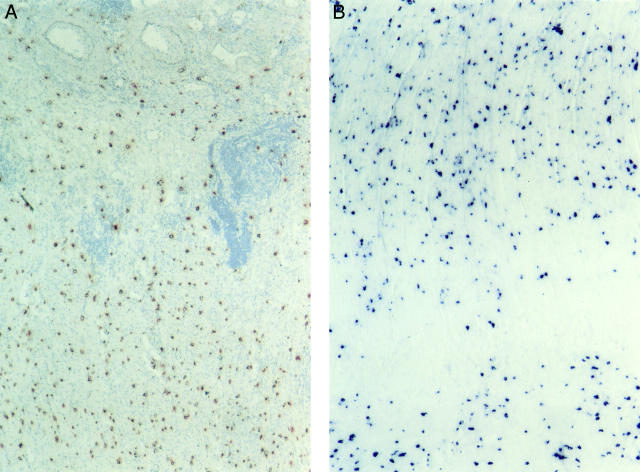
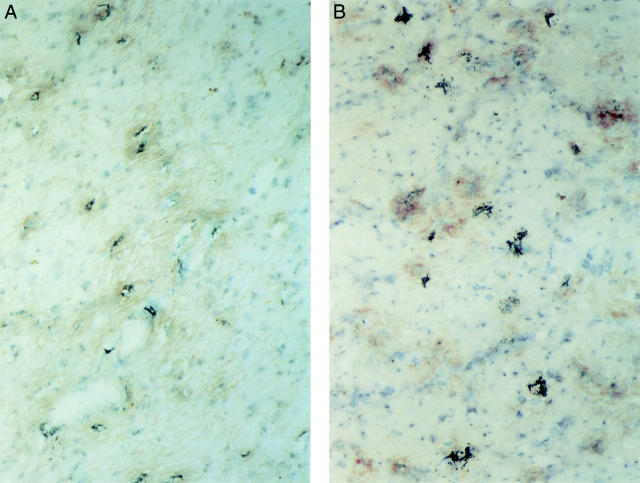
Figure 2 .
Distribution of mast cells in the muscularis propria in colonic strictures in Crohn's disease. (A) Mast cells are shown by immunohistochemical peroxidase staining for mast cell tryptase (red colour). An impressive accumulation of mast cells is found in the inner layer of the muscularis propria (lower part of the picture) beneath the submucosa (upper half of the picture). Original magnification ×100. (B) Localisation of mast cells in the inner (top two thirds of the picture) and outer (bottom) layer of the muscularis propria in an ileal stricture in Crohn's disease. Glucose oxidase staining for chymase was used to detect mast cells (dark blue colour). The highest numbers of mast cells are seen in the inner layer beneath the submucosa and in the outer layer of the muscularis propria. Original magnification ×100.
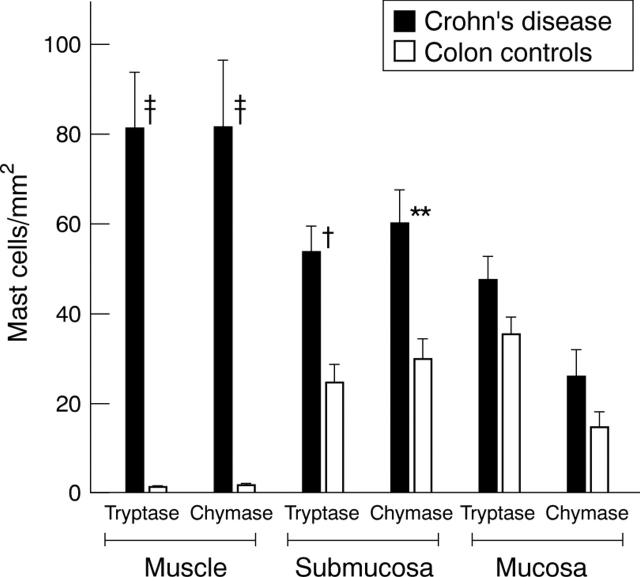
Figure 3 .
Numbers of mast cells in colonic strictures in Crohn's disease compared with normal colon. Mast cells were detected by staining for tryptase and chymase. Mast cell numbers were determined in five adjacent microscope fields in the mucosa, submucosa, and muscularis propria at 200 × magnification and normalised to 1 mm2. Mast cell numbers for the three different bowel compartments of strictured colon of Crohn's disease were compared with those of the respective compartments of normal colon: †p<0.005, ‡p<0.0005,**p<0.01.
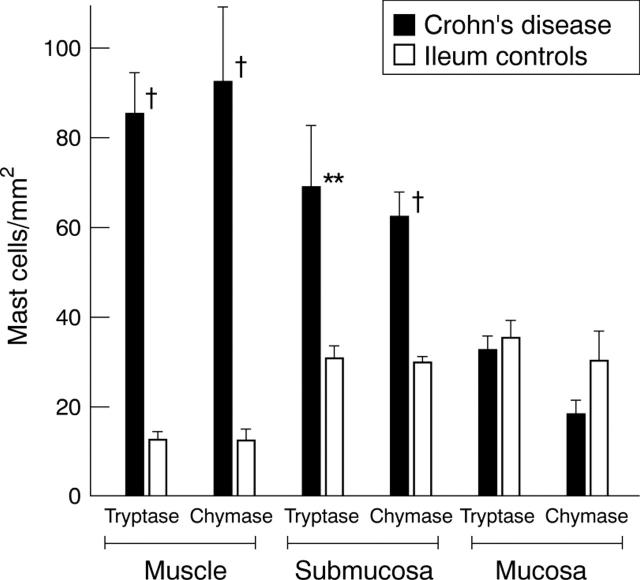
Figure 4 .
Numbers of mast cells in ileal strictures in Crohn's disease and in normal ileum. Mast cells were detected by staining for tryptase and chymase. Mast cell numbers were determined in five adjacent microscope fields in the mucosa, submucosa, and muscularis propria at 200 × magnification and normalised to 1 mm2. Mast cell numbers for the three different bowel compartments of the ileum of Crohn's disease were compared with those of the respective compartments of normal ileum: †p<0.005, **p<0.01.
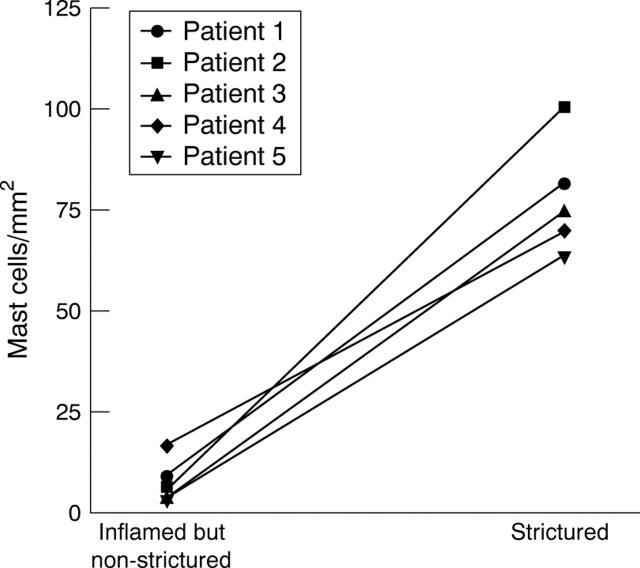
Figure 5 .
Numbers of mast cells in the muscularis propria of involved but non-strictured bowel compared with strictured bowel segments of five individual patients. Mast cells were visualised by staining for tryptase, and numbers represent cells counted in five adjacent fields at 200 × magnification normalised to 1 mm2.
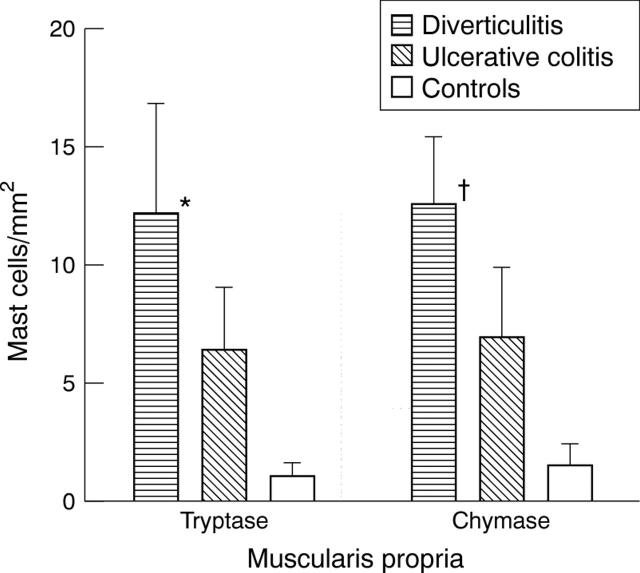
Figure 6 .
Numbers of mast cells in the muscularis propria in the colon in ulcerative colitis and sigmoid diverticulitis and in normal colon. Mast cells were detected by staining for tryptase and chymase and counted in five adjacent fields and normalised to 1 mm2. Only numbers of mast cells in sigma diverticulitis were significantly different from those in normal colon: *p<0.05, †p<0.005.
Figure 7 .
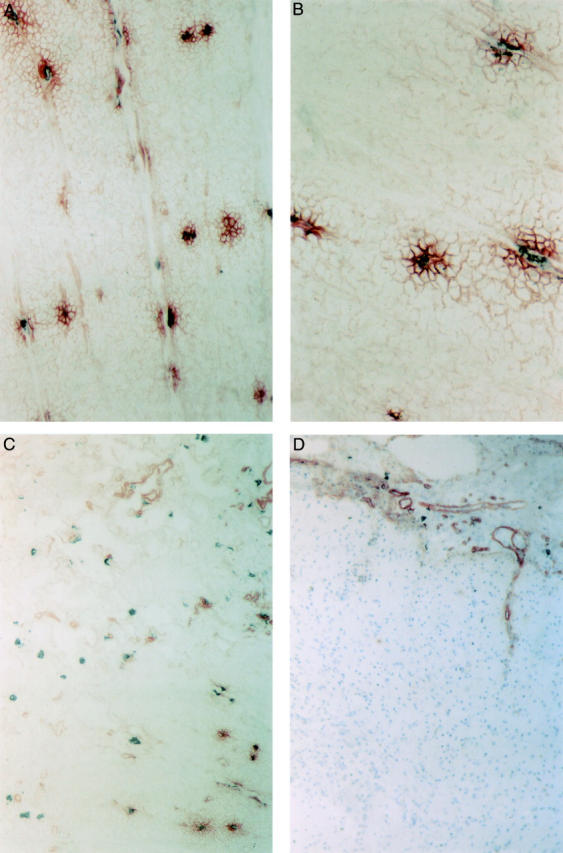
Localisation of laminin and mast cells in strictured and normal colon. Immunohistochemical double staining was used to demonstrate laminin (red colour) and mast cell chymase (grey colour). (A) Shown is the muscularis propria of a fibrotic stricture of the colon in Crohn's disease. Patches of laminin (red colour) are colocalised with mast cells (grey colour). Original magnification ×200. (B) In the muscularis propria, mast cells (grey colour) are located in the centre of laminin deposits (red colour). Original magnification ×400. (C) Distribution of laminin and mast cells in the submucosa in the same fibrotic stricture. Laminin is mainly located in the basal lamina of blood vessels where many adherent mast cells are found. A small part of the inner layer of the muscularis propria is shown in the left lower corner. Original magnification ×100. (D) Localisation of laminin in the normal colon. Laminin is found in the basal lamina of blood vessels in the submucosa (top of the picture). There are no laminin deposits and no mast cells in the muscle layer (lower part of the picture). Original magnification ×10\.
Figure 8 .
Deposition of fibronectin and vitronectin in the muscularis propria in colonic strictures in Crohn's disease. (A) Fibronectin (red colour) is diffusely distributed in the muscle layer. There is no strict colocalisation with mast cells (grey colour). Original magnification× 200. (B) Vitronectin (red colour) is spotted in the muscle layer. No colocalisation with mast cells (grey colour) is seen. Original magnification ×200.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldenborg F., Enerbäck L. The immunohistochemical demonstration of chymase and tryptase in human intestinal mast cells. Histochem J. 1994 Jul;26(7):587–596. doi: 10.1007/BF00158593. [DOI] [PubMed] [Google Scholar]
- Brown J. K., Tyler C. L., Jones C. A., Ruoss S. J., Hartmann T., Caughey G. H. Tryptase, the dominant secretory granular protein in human mast cells, is a potent mitogen for cultured dog tracheal smooth muscle cells. Am J Respir Cell Mol Biol. 1995 Aug;13(2):227–236. doi: 10.1165/ajrcmb.13.2.7626290. [DOI] [PubMed] [Google Scholar]
- Columbo M., Bochner B. S., Marone G. Human skin mast cells express functional beta 1 integrins that mediate adhesion to extracellular matrix proteins. J Immunol. 1995 Jun 1;154(11):6058–6064. [PubMed] [Google Scholar]
- Corvera C. U., Déry O., McConalogue K., Böhm S. K., Khitin L. M., Caughey G. H., Payan D. G., Bunnett N. W. Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. J Clin Invest. 1997 Sep 15;100(6):1383–1393. doi: 10.1172/JCI119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak A. M., Monahan R. A., Osage J. E., Dickersin G. R. Crohn's disease: transmission electron microscopic studies. II. Immunologic inflammatory response. Alterations of mast cells, basophils, eosinophils, and the microvasculature. Hum Pathol. 1980 Nov;11(6):606–619. doi: 10.1016/s0046-8177(80)80072-4. [DOI] [PubMed] [Google Scholar]
- Dvorak A. M., Osage J. E., Monahan R. A., Dickersin G. R. Crohn's disease: transmission electron microscopic studies. III. Target tissues. Proliferation of and injury to smooth muscle and the autonomic nervous system. Hum Pathol. 1980 Nov;11(6):620–634. doi: 10.1016/s0046-8177(80)80073-6. [DOI] [PubMed] [Google Scholar]
- Graham M. F., Diegelmann R. F., Elson C. O., Lindblad W. J., Gotschalk N., Gay S., Gay R. Collagen content and types in the intestinal strictures of Crohn's disease. Gastroenterology. 1988 Feb;94(2):257–265. doi: 10.1016/0016-5085(88)90411-8. [DOI] [PubMed] [Google Scholar]
- Gruber B. L. Mast cells: accessory cells which potentiate fibrosis. Int Rev Immunol. 1995;12(2-4):259–279. doi: 10.3109/08830189509056717. [DOI] [PubMed] [Google Scholar]
- Hartmann K., Henz B. M., Krüger-Krasagakes S., Köhl J., Burger R., Guhl S., Haase I., Lippert U., Zuberbier T. C3a and C5a stimulate chemotaxis of human mast cells. Blood. 1997 Apr 15;89(8):2863–2870. [PubMed] [Google Scholar]
- Irani A. A., Schechter N. M., Craig S. S., DeBlois G., Schwartz L. B. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofford M. W., Schwartz L. B., Schechter N. M., Yager D. R., Diegelmann R. F., Graham M. F. Cleavage of type I procollagen by human mast cell chymase initiates collagen fibril formation and generates a unique carboxyl-terminal propeptide. J Biol Chem. 1997 Mar 14;272(11):7127–7131. doi: 10.1074/jbc.272.11.7127. [DOI] [PubMed] [Google Scholar]
- Kosmehl H., Berndt A., Katenkamp D. Molecular variants of fibronectin and laminin: structure, physiological occurrence and histopathological aspects. Virchows Arch. 1996 Dec;429(6):311–322. doi: 10.1007/BF00198435. [DOI] [PubMed] [Google Scholar]
- Levi-Schaffer F., Rubinchik E. Mast cell role in fibrotic diseases. Isr J Med Sci. 1995 Jul;31(7):450–453. [PubMed] [Google Scholar]
- Matthes H., Herbst H., Schuppan D., Stallmach A., Milani S., Stein H., Riecken E. O. Cellular localization of procollagen gene transcripts in inflammatory bowel diseases. Gastroenterology. 1992 Feb;102(2):431–442. doi: 10.1016/0016-5085(92)90087-f. [DOI] [PubMed] [Google Scholar]
- Mican J. M., Di Bisceglie A. M., Fong T. L., Travis W. D., Kleiner D. E., Baker B., Metcalfe D. D. Hepatic involvement in mastocytosis: clinicopathologic correlations in 41 cases. Hepatology. 1995 Oct;22(4 Pt 1):1163–1170. doi: 10.1016/0270-9139(95)90625-8. [DOI] [PubMed] [Google Scholar]
- Miner P. B., Jr The role of the mast cell in clinical gastrointestinal disease with special reference to systemic mastocytosis. J Invest Dermatol. 1991 Mar;96(3):40S–44S. [PubMed] [Google Scholar]
- Miyake S., Yagita H., Maruyama T., Hashimoto H., Miyasaka N., Okumura K. Beta 1 integrin-mediated interaction with extracellular matrix proteins regulates cytokine gene expression in synovial fluid cells of rheumatoid arthritis patients. J Exp Med. 1993 Mar 1;177(3):863–868. doi: 10.1084/jem.177.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakianathan D. R. Extracellular matrix proteins and leukocyte function. J Leukoc Biol. 1995 May;57(5):699–702. doi: 10.1002/jlb.57.5.699a. [DOI] [PubMed] [Google Scholar]
- Paulsson M. The role of laminin in attachment, growth, and differentiation of cultured cells: a brief review. Cytotechnology. 1992;9(1-3):99–106. doi: 10.1007/BF02521736. [DOI] [PubMed] [Google Scholar]
- Pawel B. R., de Chadarévian J. P., Franco M. E. The pathology of fibrosing colonopathy of cystic fibrosis: a study of 12 cases and review of the literature. Hum Pathol. 1997 Apr;28(4):395–399. doi: 10.1016/s0046-8177(97)90025-3. [DOI] [PubMed] [Google Scholar]
- Preissner K. T. Structure and biological role of vitronectin. Annu Rev Cell Biol. 1991;7:275–310. doi: 10.1146/annurev.cb.07.110191.001423. [DOI] [PubMed] [Google Scholar]
- Richter K. K., Langberg C. W., Sung C. C., Hauer-Jensen M. Increased transforming growth factor beta (TGF-beta) immunoreactivity is independently associated with chronic injury in both consequential and primary radiation enteropathy. Int J Radiat Oncol Biol Phys. 1997 Aug 1;39(1):187–195. doi: 10.1016/s0360-3016(97)00290-3. [DOI] [PubMed] [Google Scholar]
- Sasaki M., Kleinman H. K., Huber H., Deutzmann R., Yamada Y. Laminin, a multidomain protein. The A chain has a unique globular domain and homology with the basement membrane proteoglycan and the laminin B chains. J Biol Chem. 1988 Nov 15;263(32):16536–16544. [PubMed] [Google Scholar]
- Thompson H. L., Burbelo P. D., Gabriel G., Yamada Y., Metcalfe D. D. Murine mast cells synthesize basement membrane components. A potential role in early fibrosis. J Clin Invest. 1991 Feb;87(2):619–623. doi: 10.1172/JCI115038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H. L., Burbelo P. D., Metcalfe D. D. Regulation of adhesion of mouse bone marrow-derived mast cells to laminin. J Immunol. 1990 Nov 15;145(10):3425–3431. [PubMed] [Google Scholar]
- Thompson H. L., Burbelo P. D., Segui-Real B., Yamada Y., Metcalfe D. D. Laminin promotes mast cell attachment. J Immunol. 1989 Oct 1;143(7):2323–2327. [PubMed] [Google Scholar]
- Thompson H. L., Burbelo P. D., Yamada Y., Kleinman H. K., Metcalfe D. D. Identification of an amino acid sequence in the laminin A chain mediating mast cell attachment and spreading. Immunology. 1991 Jan;72(1):144–149. [PMC free article] [PubMed] [Google Scholar]
- Tryggvason K. The laminin family. Curr Opin Cell Biol. 1993 Oct;5(5):877–882. doi: 10.1016/0955-0674(93)90038-r. [DOI] [PubMed] [Google Scholar]
- Vermillion D. L., Huizinga J. D., Riddell R. H., Collins S. M. Altered small intestinal smooth muscle function in Crohn's disease. Gastroenterology. 1993 Jun;104(6):1692–1699. doi: 10.1016/0016-5085(93)90647-u. [DOI] [PubMed] [Google Scholar]
- Vliagoftis H., Metcalfe D. D. Characterization of adhesive interactions between mast cells and laminin isoforms: evidence of a principal role for alpha 6 integrin. Immunology. 1997 Dec;92(4):553–560. doi: 10.1046/j.1365-2567.1997.00368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]