Abstract
In the presence of ascorbate/H2O2, ATP–Fe2+ or AMP-PNP–Fe2+ complexes act as affinity cleavage reagents, mediating selective cleavage of the alpha subunit of Na,K-ATPase at high affinity ATP–Mg2+ sites. The cleavages reveal contact points of Fe2+ or Mg2+ ions. In E1 and E1Na conformations, two major cleavages are detected within the conserved 708TGDGVNDSPALKK sequence (at V712 and nearby), and one (E1Na) or two (E1) minor cleavages near V440. In media containing sodium and ATP, Fe2+ substitutes for Mg2+ in activating phosphorylation and ATP hydrolysis. In the E1P conformation, cleavages are the same as in E1. Fe2+ is not bound tightly. By contrast, in the E2P conformation, the pattern is different. A major cleavage occurs near the conserved sequence 212TGES, whereas those in TGDGVNDSPALKK are less prominent. Fe2+ is bound very tightly. On E2P hydrolysis, the Fe2+ dissociates. The results are consistent with E1↔E2 conformation-dependent movements of cytoplasmic domains and sites for Pi and Mg2+ ions, inferred from previous Fe-cleavage experiments. Furthermore, these concepts fit well with the crystal structure of Ca-ATPase [Toyoshima, C., Nakasako, M., Nomura, H. & Ogawa, H. (2000) Nature (London) 405, 647–655]. Altered ligation of Mg2+ ions in E2P may be crucial in facilitating nucleophilic attack of water on the O—P bond. Mg2+ ions may play a similar role in all P-type pumps. As affinity cleavage reagents, ATP–Fe2+ or other nucleotide–Fe2+ complexes could be widely used to investigate nucleotide binding proteins.
Keywords: energy transduction mechanism
A long series of experiments established the Post–Albers kinetic mechanism of Na,K-ATPase and related P-type cation pumps (1–3). Active Na+ and K+ transport involves (i) Nacyt-dependent phosphorylation from ATP, and Na+ occlusion, E1→E1P(Na); (ii) Na+ transport outward across the membrane coupled to E1P→E2P; (iii) Kexc-activated dephosphorylation, and occlusion, E2P→E2(K); and (iv) K+ transport inward across the membrane coupled to E2(K)→E1, accelerated by ATP acting with low affinity. For other pumps, steps i or iii are activated by the appropriate cations, which are transported in steps ii or iv.
Despite our extensive knowledge of function, a proper understanding of active transport cannot be achieved without knowledge of molecular structure. In this regard, the recent publication of the 2.6-Å crystal structure of sarcoplasmic reticulum Ca-ATPase is an event of unparalleled importance (4). The structure confirms the existence of ten transmembrane helices deduced for Ca, Na,K−, H,K−, and H-pumps by biochemical techniques (5) and reveals several unexpected features, including distortion of the M4 and M6 membrane-spanning helices involved in occluding Ca ions. The details of Ca occlusion sites fit well with those deduced in extensive mutagenesis studies (3, 6). The cytoplasmic sector of the pump is divided into three domains, two domains N (nucleotide) and P (phosphorylation) within the loop between M4 and M5, well separated from a third A (actuator or anchor) domain containing the loop between M2 and M3 and the segment leading into M1. The fold of the P domain is like that of haloacid dehydogenase and related proteins with homologies to P-type pumps in conserved cytoplasmic sequences (7, 8). Comparison of the crystal structure (an E1⋅Ca conformation) with cryoelectron microscope images of Ca-ATPase in both E1 or E2 conformations (9), suggested that, in the change from E1 to E2, domain A makes contact with the P/N domain (see below). Presumably, the tertiary structures of other P-type pumps will resemble that of Ca-ATPase, particularly within the cytoplasmic domains, but will show detailed differences related to the cation specificities, and for Na,K-ATPase and H,K-ATPase to the presence of a β subunit .
Important as it is, the crystal structure of a pump in one conformation cannot provide full structural information on other conformations. Recently, we described a technique of specific oxidative cleavage of renal Na,K-ATPase, which utilizes Fe2+/ascorbate/H2O2 and provides unique information on spatial organization in E1 and E2 conformations (refs. 10–12, and see also ref. 13 for specific Cu-mediated oxidative cleavage). Peptide bonds close to a bound Fe2+ ion are cleaved by locally generated OH⋅ radicals or by a reactive Fe2+-peroxyl intermediate. Because several peptide bonds are cleaved from the same Fe2+ site, these cleavage positions must be in proximity in the native protein. In E2 or E2(K) forms, five or six fragments were observed whereas in E1 or E1Na forms only two fragments were observed. Cleavage positions were identified either exactly or approximately. Significantly, four cleavages are located at or near conserved cytoplasmic sequences, TGES in the A domain, and CSDK, MVTGD and TGDGVNDSPALKK in the P domain. Two cleavages are located near a sequence HFIH close to the entrance of M3 and the entrance to M1. These findings led to a proposal that, in the E2 conformations, a domain formed by the cytoplasmic loop between M2 and M3 and cytoplasmic segment leading into M1 interacts with a domain formed by the cytoplasmic loop between M4 and M5, via residues in the conserved sequences whereas, in E1 conformations, the two domains are well separated (10, 12). This evidence for large conformation-dependent domain movements fits very well with the inference drawn from the Ca-ATPase crystal structure (4). Subsequent observations on cleavages of phosphorylated enzyme and inhibitory effects of Pi,Mg2+,ouabain and vanadate,Mg2+ suggested that the domain movements occur also in the E1P↔E2P transition, and the interactions occur within the phosphorylation site. Noncovalently bound Pi or covalently bound phosphate were proposed to interact with residues near the CSDK and MVTGD sequences, and Mg2+ ions with TGES and TGDGVNDAPALKK sequences, and also with the bound phosphate (11). The cleavage experiments were interpreted simply by assuming the existence of a single Fe2+ site. However, an alternative hypothesis that cleavage occurs from two Fe2+ sites, one of which includes the points near M1 and HFIH and does not change in E1 and E2 forms, whereas the other includes the points at ESE, near CSDK, near MVTGD and at VNDS and exists only in E2 forms, could not be excluded (11). The Ca-ATPase structure shows that the conserved sequences in the P domain are close to each other but are far (>40 Å) from 255EFGE, the Ca-ATPase equivalent of the HFIH sequence near M3. This new information makes a two-Fe2+ site mechanism a likely possibility. It does not affect the basic conclusion on the domain movements accompanying the E1→E2 transition.
The present work takes the cleavage technique in a new direction. We now demonstrate that Fe2+ complexes of adenine nucleotides bind to and catalyze specific cleavages in the ATP sites. Furthermore, Fe2+ substitutes for Mg2+ ions in activating phosphorylation, allowing detection of cleavages within the active site in different phosphorylated conformations. These findings provide novel information on the reaction mechanism.
Materials and Methods
Materials.
For SDS/PAGE, all reagents were electrophoresis-grade from Bio-Rad. Tris (ultra pure) was from Bio Lab, Jerusalem. L(+) ascorbic acid (cat. 100127) and 30% H2O2 (cat. 822287) were from Merck. Desferrioxamine mesylate (Desferal) (D9533), ATP(Na)2 (A2383) and AMP-PNP (Li salt) (A2647), FITC (F4274), oligomycin (O4876), and ouabain (O3125) were from Sigma. Other reagents were of analytical grade. Choline chloride was recrystallized from ethanol. ATP(Na)2 was converted to the Tris salt by passage through a column of Dowex-Tris.
Enzyme Preparation.
Na,K-ATPase (13–18 units/mg protein) was prepared from pig kidneys, assayed, and stored at −20°C in a solution of 250 mM sucrose, 25 mM histidine (pH 7.4), and 1 mM EDTA, as described in ref. 14. Before use, membranes were washed twice and suspended in a buffer solution containing 10 mM Tris⋅HCl (pH 7.2). FITC-labeled enzyme was prepared as described in ref. 15 by incubation with 20 μM FITC, at pH 9, for 4 h at 20°C.
Cleavage Reaction.
Membrane suspensions (0.25 mg/ml) were suspended in the buffer containing also choline chloride 300 mM, NaCl or RbCl 130 mM or 30 mM, respectively, in lanes marked Cont. Rb or Cont. Na, and other solutions as indicated. The enzyme was incubated at 20°C with freshly prepared solutions of 5 mM ascorbate (Tris) plus 5 mM H2O2, 5 μM FeSO4 and ATP(Tris) or AMP-PNP (Li salt) at indicated concentrations in a total volume of 30 μl. To arrest the reaction, 20 μl of the gel sample buffer containing also 5 mM EDTA and 5 mM Desferal was added, and samples were applied to gels.
Gel Electrophoresis, Blotting to Poly(vinylidene difluoride), Immunoblots, and Sequencing.
Procedures for running of 10% Tricine SDS/PAGE, including precautions before sequencing, electroblotting to poly(vinylidene difluoride) paper, immunoblots, and microsequencing of fragments have been described in detail (16, 17). All immunoblots were probed with anti-KETYY, which recognizes the C terminus of the α subunit. Where fragments were to be sequenced, cleaved enzyme (1 mg/ml) was extracted with the nonionic detergent C12E10 (polyoxyethylene 10-laurylether) to remove contaminant proteins, before application to long 10% gels (see ref. 10).
Results
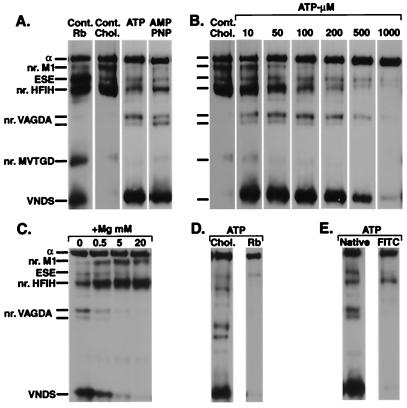
Fig. 1 A–E presents evidence that the Fe2+ complexes of ATP or the nonhydrolyzable analogue AMP-PNP act as specific affinity cleavage agents in the ATP-Mg2+ sites. Unless indicated otherwise, the enzyme was suspended in a medium of high ionic strength (containing 300 mM choline chloride), lacking Na+ or K+ ions. In this condition, the enzyme is mainly in an E1 conformation and binds ATP or AMP-PNP with high affinity. In Fig. 1A, the lanes marked Cont. Rb and Cont Chol. display the standard pattern of fragments produced by Fe2+/ascorbate/H2O2 in media containing Rb+ ions (10 mM) or choline chloride (E2(Rb) or E1 conformations, respectively), together with their exact or approximate cleavage positions, as determined previously (10, 12). These fragments, which are products of cleavages catalyzed by Fe2+ ions bound at their site(s) without ATP, serve as a convenient reference for comparison with those produced with ATP or AMP-PNP. In the presence of 30 μM ATP or AMP-PNP and Fe2+/ascorbate/H2O2, two phenomena were observed (Fig. 1A). First, the fragments referred to as nr.M1 and HFIH were largely suppressed. Second, new fragments appeared, a broad band close to the known VNDS cleavage position, and two much less prominent fragments marked as nr.VAGDA. In many gels the broad band was resolved into two fragments (apparent molecular mass, 26.5 and 25 kDa, respectively; see Figs. 2 and 3). The position of the upper of the two minor bands (apparent molecular mass, 55.3 and 53.0 kDa, respectively) is close to that of a known chymotryptic fragment with N terminus V440 (11), and, accordingly, these fragments are referred to as near VAGDA. Adenine nucleotides form complexes with Fe3+ and Fe2+ ions, and the complexed Fe3+ and Fe2+ ions catalyze efficient generation of OH⋅ radicals by the Fenton reaction (18–20). Thus, an economical explanation of the findings in Fig. 1A is that ATP or AMP-PNP chelate the free Fe2+ ions, thus precluding binding and cleavage at the site near M1 and M3, whereas the ATP–Fe2+ or AMP-PNP–Fe2+ complexes bind to the high affinity ATP site where cleavage occurs. This hypothesis was tested further and confirmed (Fig. 1 B–E). As seen in Fig. 1B, when the ATP concentration was varied over a wide range (10–1,000 μM) with a fixed concentration of 5 μM Fe2+ ions, the cleavages nr.M1 and HFIH were progressively suppressed, and the new fragments at VNDS and nr.VAGDA appeared. However, at a large excess of ATP (1,000 μM), the cleavages at VNDS and nr.VAGDA were also completely suppressed. The latter behavior is explained most simply by assuming that an excess of uncomplexed ATP (995 μM) competes with the ATP–Fe2+ complex (maximal concentration 5 μM) in the site. ATP binds Mg2+ ions, and thus excess of Mg2+ ions should displace Fe2+ from the ATP and suppress the cleavages from the ATP site. This phenomenon is seen clearly in Fig. 1C. Notice also that, as the Mg2+ concentration was raised, the cleavages nr.M1 and HFIH were restored. The latter effect indicates that, as Mg2+ displaces the Fe2+ ions from the ATP, the newly released Fe2+ rebinds to and catalyzes cleavages in its site nr.M1 and M3, and in addition Mg2+ ions do not interfere with cleavages by Fe2+ as we reported previously (11). Mn2+ and Co2+ also interfered with the cleavages, at about 4-fold lower concentrations than Mg2+. In media containing K+ or congener such as Rb+ ions, the enzyme is stabilized in the E2(K) or E2(Rb) conformation, and ATP binds only with a low affinity (21). Fig. 1D shows that essentially no cleavages occurred in the medium containing RbCl, implying that the ATP–Fe2+ complex also does not bind in this condition. Finally, cleavages induced by the ATP–Fe2+ complex were abolished in enzyme selectively modified with FITC, whereas those at the site near M1 and M3 were unaffected (Fig. 1E). FITC prevents binding of ATP to Na,K-ATPase, by specifically modifying K501 within the ATP binding site (15, 22). Thus, Fig. 1E provides the most direct demonstration that the cleavages occur in the ATP site. Similar phenomena to those in Fig. 1 B–E were observed with AMP-PNP, and lower concentrations were required to suppress cleavages at the Fe2+ site near M1 and M3, implying that the Fe2+ ion binds more tightly to AMP-PNP than to ATP.
Figure 1.
Cleavage of Na,K-ATPase mediated by ATP–Fe2+ or AMP-PNP–Fe2+ complexes. The enzyme was suspended in the medium containing 300 mM choline chloride, or other salts as indicated, and incubated with 5 mM ascorbate/H2O2, 5 μM FeSO4 as follows. (A) In lanes designated Cont. Rb and Cont. choline, ATP was omitted; in the other lanes, 30 μM ATP or AMP-PNP were added (5 min incubation); (B) ATP as indicated, 4 min incubation; (C) 166 μM ATP, MgCl2 as indicated, 5 min incubation; (D) 500 μM ATP, 10 min incubation; (E) 500 μM ATP, 30 min incubation.
Figure 2.
Cleavage of Na,K-ATPase in conditions permitting phosphorylation. The enzyme was suspended in the medium containing 130 mM NaCl or other salts, and incubated with 5 mM ascorbate/H2O2, 5 μM FeSO4 as follows: (A) 166 μM ATP and indicated times; (B) 40 μM AMP-PNP and indicated times; (C) 500 μM ATP, 10 min incubation; (D) 500 μM ATP, oligomycin, 200 μg/ml, 5 min incubation; (E) 20 μM AMP-PNP, 300 mM choline chloride or 300 mM NaCl, 5 min incubation.
Figure 3.
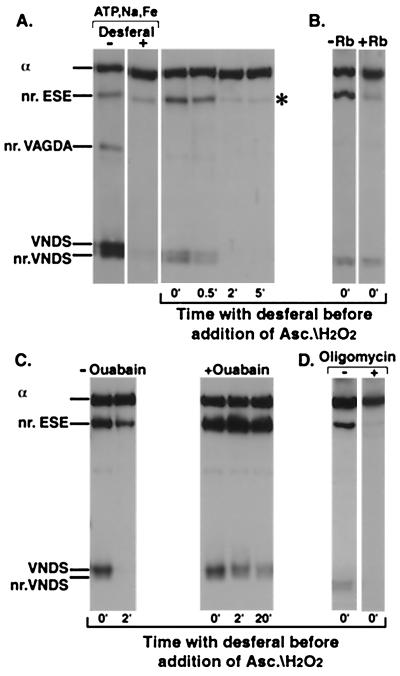
Cleavage of Na,K-ATPase in the E2P conformation. (A) Lanes 1 and 2 only (Control). The enzyme was suspended at 0°C in a medium containing 130 mM NaCl, 1,000 μM ATP, 50 μM FeSO4, without or with 4 mM Desferal, and was incubated with 20 mM ascorbate/H2O2 for 5 min. (A–D) All other lanes. The enzyme was suspended at 0°C in a medium containing 130 mM NaCl, 1,000 μM ATP. FeSO4 (50 μM) was added to initiate formation of the phosphoenzyme. After 1 min incubation, 4 mM Desferal was added to terminate phosphoenzyme formation and trap free Fe2+. Ascorbate/H2O2 (20 mM) was added either together with the Desferal or later at indicated times. After 1 min further incubation to generate the cleavages, the gel sample buffer was added to stop the reaction. (A) Lanes 3–6, stability of tightly bound Fe2+. Ascorbate/H2O2 was added at indicated times after Desferal. (B) Dephosphorylation of E2P. Without or with 20 mM RbCl added with the Desferal/ascorbate/H2O2. (C) Inhibition of E2P hydrolysis. Ouabain (1 mM) was added after the incubation with Na+/ATP/Fe2+ to inhibit the phosphoenzyme, and, 1 min later, Desferal was added. Ascorbate/H2O2 was added together with the Desferal (0 min) or after the indicated times (2 min and 20 min). (D) Blocking of E1P-E2P; 200 μg/ml oligomycin was present in the incubation medium with Na+/ATP/Fe2+
It has been known for many years that Fe2+ ions substitute for Mg2+ ions as activators of Na,K-ATPase activity (23) and covalent phosphorylation of the protein (24). The experiments in Fig. 1 suggest strongly that Fe2+ in the ATP–Fe2+ complex replaces Mg2+ of the normal ATP–Mg2+ complex. Thus, it was of interest to look at conditions that might allow Fe2+-catalyzed phosphorylation and ATP hydrolysis, in a medium containing Na+/ATP/Fe2+, but lacking Mg2+ ions (Fig. 2). Fig. 2A presents a time course of cleavage in the medium containing 150 mM Na+, 5 μM Fe2+, and 500 μM ATP (at these concentrations of ATP and Fe2+, cleavages near M1 and M3 are largely suppressed). One striking observation is that a new band appeared near the ESE position (asterisk, compare with Cont. Rb) in addition to the band at VNDS (resolved into two fragments), and a single band at the nr.VAGDA position. With AMP-PNP, the extra fragment near ESE was not observed (Fig. 2B). Appearance of the fragment near ESE only in the presence of Na+ ions, and with ATP but not with the nonhydrolyzable analogue, AMP-PNP, suggests strongly that it is formed by cleavage of a phosphoenzyme. Cleavages at all three positions near ESE, VAGDA, and VNDS were abolished in FITC-labeled enzyme (Fig. 2C), indicating the requirement for binding the ATP–Fe2+ complex. The presence of oligomycin prevented appearance of the extra band near ESE (asterisk) but not of the other fragments (Fig. 2D). Oligomycin blocks the E1P→E2P conformational change, stabilizing E1P (25). Thus, an important implication is that cleavage near ESE occurs only in the E2P form (see below). An additional observation in Fig. 2A is that only one minor fragment nr.VAGDA is produced in the Na+-rich medium, rather than the two fragments in the choline chloride medium of Fig. 1. Because the experiments in Fig. 1 and 2 were not all done at identical ionic strengths, we compared cleavages at 300 mM NaCl or choline chloride by using the AMP-PNP–Fe2+ complex (Fig. 2E). The experiment shows either one or two bands near the VAGDA location, in the NaCl and choline chloride media, respectively. Because this difference was observed with the AMP-PNP–Fe2+ complex, it cannot be associated with phosphorylation, but must reflect a structural difference in the ATP binding site in media containing NaCl or choline chloride (see Discussion).
The results in Fig. 2 are fully consistent with the assumption that, in the Na+-containing medium, Fe2+ ions bound to ATP substitute for Mg2+ ions in activating phosphorylation and ATP hydrolysis. Although the experiment with oligomycin in Fig. 2D implies that the cleavage near ESE occurs only in E2P, it does not allow one to determine whether this cleavage is the only one in E2P, because other enzyme conformations, E1 and E1P, are also present. Therefore, we designed an experiment to isolate the E2P conformation kinetically and determine its cleavage pattern (Fig. 3). The experiment is based on Fukushima and Post's proposal that Mg2+ ions are tightly bound to phosphoenzymes (24). Tight binding to E1P or E2P of Co2+ or Mn2+ ions, which also substitute for Mg2+ ions, was later demonstrated directly (26, 27). The enzyme was first incubated in the Na+/ATP/Fe2+ medium to allow phosphorylation, and then a large excess of the specific Fe2+ chelator, Desferal, was added to remove free Fe2+ from the medium and stop further phosphorylation. Ascorbate/H2O2 were added together with or after the Desferal, and the mixture was incubated for a fixed period before addition of gel buffer, which denatures the protein, chelates released Fe2+, and stops further cleavage. If Fe2+ ions activate phosphorylation, become tightly bound, and are still accessible to ascorbate/H2O2, then cleavages could occur even in the presence of the Fe2+ chelator. An additional condition is that the rate of cleavage should exceed the rate of E2P hydrolysis, and, therefore, to reduce the rate of E2P hydrolysis, the experiment was done at OoC. In Fig. 3A, the control experiment (− or + Desferal) shows that addition of Desferal before incubation with Na+/ATP/Fe2+ and then ascorbate/H2O2 essentially suppressed all cleavages, showing that the chelator reduced free Fe2+ to a low concentration. Remarkably, when the enzyme was incubated first with Na+/ATP/Fe2+ and Desferal/ascorbate/H2O2 were added subsequently, the fragment near ESE (asterisk) and smaller amounts of the fragments near VNDS were clearly detected (Fig. 3A, lane 3, marked 0′). Introduction of a time interval between the addition of Desferal and ascorbate/H2O2 led to a rapid decline in the yield of the fragments, with a half-time of roughly half a minute (Fig. 3A, lanes 4–6, 0.5 min, 2 min, and 5 min). In all experiments in Fig. 3, the incubation time with ascorbate/H2O2 was 1 min; longer incubations did not change yields of fragments (not shown). Tentatively, one could conclude that the assumptions underlying the experiment in Fig. 3A are valid and, presumably, that spontaneous hydrolysis of E2P released the Fe2+, so preventing cleavage. The additional experiments in Fig. 3 B–D examined the hypothesis in further detail. K+ ions catalyze rapid hydrolysis of E2P formed by Na+/ATP/Mg2+, and ouabain stabilizes E2P by greatly reducing the rate of E2P hydrolysis (28). E2P formed with Na+/ATP/Fe2+ is sensitive to K+ ions and is stabilized by ouabain (R. L. Post, personal communication). Fig. 3B shows that cleavages seen in the conditions of Fig. 3A were not seen when Rb+ ions were added together with the Desferal/ascorbate/H2O2, consistent with rapid E2P hydrolysis and release of the bound Fe2+ ions. In the experiment of Fig. 3C, ouabain was added after the preincubation with Na+/ATP/Fe2+, then Desferal, and ascorbate/H2O2 at the indicated time intervals (0 min, 2 min, or 20 min). Clearly, ouabain stabilized the intermediate because the yield of the fragment near ESE increased and was unaffected by an interval of up to 20 min between addition of Desferal and ascorbate/H2O2. Finally (Fig. 3D), if oligomycin was present in the preincubation medium with ATP/Na+/Fe2+, no cleavages were observed after addition of Desferal/ascorbate/H2O2. This result shows both that it is E2P that undergoes cleavage in these conditions, and also that Fe2+ is bound less tightly in the E1P conformation stabilized by oligomycin. Overall, the data in Fig. 3 confirms the prediction of cleavage of the E2P conformation mediated by tightly bound Fe2+. The most striking feature is that in E2P the protein is cut preferentially near ESE whereas cleavages at or near VNDS are less prominent (see Discussion).
Gels of fragments produced in the conditions of Figs. 1B or 3C. were stained with Coomassie, and fragments were transferred to poly(vinylidene difluoride) paper for sequencing. In conditions of Fig. 1B (500 μM ATP), the major fragments had apparent molecular mass values of 25.5 and 81 kDa, respectively, and gave N-terminal sequences 712VNDS and 1GRDK, respectively, indicating that these are complementary fragments. Two major fragments were produced in conditions of Fig. 3C, with apparent molecular mass values 78.9 and 22.9 kDa, respectively. The 78.9-kDa fragment near ESE gave no sequence because of a blocked N terminus whereas the 22.9-kDa fragment gave GRDK, as expected, for the complementary fragment. Minor fragments seen in immunoblots were not detected in Coomassie-stained gels because of the lower sensitivity. No major fragments were detected in addition to those detected in immunoblots, excluding the possibility of cleavage at more than one position per polypeptide chain.
Discussion
Fig. 4 presents schematic models depicting residues ligating the Fe2+ ion, and the γ phosphate of ATP or covalently bound phosphate, in the different conformations. The properties of cleavages fit well with the assumption that Fe2+ substitutes for Mg2+ in the ATP-Mg site, and activates phosphorylation and ATP hydrolysis. Thus, the conclusions concerning Fe2+ should also be valid for Mg2+ ions. The models are based on the current and previous cleavage experiments and fit well with the crystal structures of Ca-ATPase and the HAD (L-2-haloacid dehalogenase) and CheY response regulator proteins with a homologous fold of the phosphorylation domain (4, 7, 8). The organization of cytoplasmic loops into N, P, and A domains, and the relative positions of residues ligating the phosphate group or Fe2+ (Mg2+) in the E1 conformations, are drawn to be similar to that in Ca-ATPase. The current experiments strongly support the concept of conformation-dependent domain movements. Overall, one can infer that the A domain docks onto the P domain in E2(K) and E2P and the N domain docks onto the P domain in E1 or E1P, the A domain being displaced to the side. Furthermore the experiments provide new information on changes in Mg2+ binding accompanying the E1P→E2P conformational transition. Presumably, the concepts in Fig. 4 apply to other P-type pumps.
Figure 4.
Schematic models of the active site with bound Fe2+ in different conformations.
E1Na.
ATP–Fe2+ or AMP-PNP–Fe2+ complexes bind in the high affinity ATP-Mg2+ site in E1 or E1⋅Na conformations. Bound Fe2+ is ligated to the β and γ phosphates and the N7 of the purine ring of ATP, to D710 and D714 of the TGDGVNDS sequence in the P domain, and, further away, to a sequence near VAGDA in the N domain. In Ca-ATPase, the residues equivalent to D710 and D714 are located on one side of a triad, with the phosphorylated D369 at the apex (4), and in CheY Mg2+ binds to the residues equivalent to D710 and D714 (8). For bound Fe2+, this arrangement explains well the two major cleavages at and just beyond V712. (Fig. 1, choline medium, or Fig. 2, Na-medium with AMP-PNP). The less prominent cleavages near VAGDA (Fig. 1 and 2E) imply proximity of this segment with D710 and D714 when ATP is bound. Therefore, the Fe2+ makes contact also with the purine ring because it is this part of the ATP molecule that interacts with the N domain (4). Various transition metals, including Mn2+, which substitutes for Mg2+, interact with the purine ring as well as with β and γ phosphates of ATP (29). The sequence 440VAGDA of Na,K-ATPase aligns with the sequence 438EATET of Ca-ATPase, which contains a residue T441 shown to lie within the ATP binding pocket (4). Thus, the cleavages show that the N domain must come into proximity or dock onto the P domain. The γ phosphate of ATP interacts with D369 as well as with the conserved K691 and T610 of the MVTGD sequence as proposed for Ca-ATPase and CheY. This arrangement is consistent with lack of cleavages by the ATP–Fe2+ complex at these positions, indicating that bound Fe2+ (Mg2+) is not in direct contact with D369, T610, or K691. A suggestion that Mg2+ ions bind to D586 of DPPR and in the MVTGD sequence (30) is not supported by the present data or the crystal structure of Ca-ATPase. The A domain is separated and oriented away from the N and P domains, precluding cleavage by ATP–Fe2+ at TGES.
E1P.
Fe2+ is still bound to D710 and D714, near VAGDA, and to covalently bound phosphate, and the other features are the same as in E1Na, thus explaining the same cleavages in E1P and E1Na (Fig. 2D). Fe2+ is not very tightly bound (Fig. 3D). In this respect, Fe2+ is different from Mg2+, Mn2+, and Co2+ ions, which are tightly bound also in E1P (24, 26, 27).
E2P.
In E2P, the major cleavage occurs near the conserved TGES, whereas those at or near VNDS are less prominent. Fe2+ is very tightly bound, and on hydrolysis of E2P, the bound Fe2+ ion dissociates (Fig. 3 A and B). The model depicts a large movement and reorientation of domain A toward the P domain, as predicted from the previous cleavage experiments (10, 11) and the inferred structure of Ca-ATPase in the E2 conformation (4). E214 in TGES sequence in the A domain makes contact with tightly bound Fe2+, explaining the major cleavages near TGES, whereas D710 and D714 are somewhat displaced to account for the less prominent cleavage at this position. The model fits well with our previous suggestion (11) that Mg2+ is ligated by residues in both the VNDS and TGES sequences and phosphate by residues in the CSDK and MVTGD sequences, based on inhibitory effects of Pi,Mg2+, Pi,Mg2+,ouabain, or vanadate,Mg2+ on cleavage catalyzed by Fe2+ without ATP.
E2(K).
An Fe2+ ion is bound in the absence of ATP (or at low ATP concentrations) as concluded from earlier work (see figures 5 and 6 of ref. 11). At high ATP concentrations, free Fe2+ is chelated in the ATP–Fe2+ complex, which is not bound in E2(Rb) (Fig. 1). The residues within TGES in the A domain and CSDK, MVTGD, and VNDSPALKK in the P domain are in proximity, and their interactions appear to maintain the contacts between the A and P domains. In the inferred structure of Ca-ATPase in an E2 state, the A domain docks onto the P domain, and the TGES and MVTGD sequences are indeed close to each other (4).
Consequences of Domain Movements Accompanying E1→E2 Conformational Transitions.
Movement of the A domain toward the P/N domain, accompanying E1P→E2P, must be coupled to movements of transmembrane segments (M4, M5, M6, M8, ?) that release Na+ ions at the exterior. Oligomycin, which is hydrophobic and blocks release of Na+ ions (31), could interfere with movements of transmembrane segments, which would, in turn, prevent the A to P domain docking. The ATP–Mg2+ complex accelerates the rate of the E2(K)→E1Na with low affinity and binds with high affinity to E1Na, presumably to the N and P domains, so forcing separation of A and P domains. The reopening of the cytoplasmic domains should be associated with the opposite movement of transmembrane segments and release of K+ ions to the interior.
Assuming that the change in ligation of the Fe2+ ion in the E1, E1P, and E2P states described here are paralleled by those of the Mg2+ ion, the phenomenon must have an important mechanistic significance. Mg2+ ions are required for phosphorylation and facilitate nucleophilic attack by the carboxylate of D369 on the γ phosphate of ATP, presumably by shielding the negative charge and raising electrophilicity of the phosphorus atom. E1P and E2P have different properties, which are essential features of the pump (1). E1P, like ATP, has a high free energy of hydrolysis and can transfer its phosphate to ADP, but it is not readily hydrolyzed. E2P has a low free energy of hydrolysis and cannot transfer its phosphate to ADP, but it is more readily hydrolyzed, rapidly so when K+ ions are bound.
Tight binding of Mg2+ ions in E2P is required for its normal reactivity to water (24). An important implication of the altered ligation of Mg2+ in E2P is that geometry of ligands surrounding the bound phosphate should change. A change in geometry could be crucial for facilitating nucleophilic attack by water on the phosphorus atom of the O—P bond. ATP hydrolysis occurs with overall retention of stereochemical configuration of released phosphate, and the simple explanation is that both phosphorylation and dephosphorylation reactions involve “in-line” nucleophilic reactions, via penta-coordinate transition state intermediates, each with inversion of configuration (32). K+ ions, which greatly accelerate hydrolysis, act at a distance, and a likely mechanism involves induction of an appropriate configuration for “in-line” nucleophilic attack by water on the phosphorus. Repke and Schon have proposed that pseudorotation about the C—O—P bonds is necessary to labilize the O—P bond to water (33). This mechanism also implies a change in geometry of the phosphate ligands. The closed domain structure in E2P is compatible with the proposal, based on effects of organic solvents, that the environment of the C—O—P bond is hydrophobic (34). A hydrophobic environment should amplify shielding by Mg2+ ions of negative charge on the phosphate oxygens and also facilitate hydrolysis of E2P. On hydrolysis of E2P, the Mg2+ ion dissociates.
A Na-Dependent Change in the Nucleotide Binding Domain.
The small but detectable difference in cleavage near VAGDA in the Na+-rich and choline-rich media (one or two fragments, respectively, Fig. 2E) is of interest, for in both conditions the enzyme is an E1 form with high ATP affinity. The result shows that ligation of the Fe2+ (Mg2+) ion bound to the ATP changed. A small difference in fluorescence of FITC bound at K501 between E1 and E1Na states has also been reported (35). These findings indicate that Na+ ions, bound within transmembrane segments, induce a long range conformational change in the N domain. Although this rearrangement within the ATP site is minor, by comparison with the domain movements in E1↔E2 transitions, it represents an essential step in which Na+ ions trigger phosphorylation. Possibly, the Na-dependent change in the N domain brings D369 into closer proximity with the γ phosphate of ATP.
Additional Applications of Nucleotide–Fe2+ Complexes as Affinity Cleavage Reagents.
(i) For Na,K-ATPase, an additional use of adenine nucleotide–Fe2+ complexes concerns recent proposals that there are both high and low affinity ATP binding sites on the enzyme (36, 37). Preliminary cleavage experiments utilizing high concentrations (500 μM) of ATP–Fe2+ and AMP-PNP–Fe2+ complexes have not provided evidence for more than one ATP binding site (G.P. and S.J.D.K., unpublished results). (ii) Because of the simplicity of the cleavage technique, Fe2+ complexes of ATP, GTP, or other nucleotides may become very useful as affinity cleavage reagents for a variety of nucleotide binding proteins, for mapping nucleotide sites, analyzing modifications or mutations, etc. In addition, for cases in which Fe2+ substitutes for Mg2+ in promoting enzyme activity, the associated structural changes may be detected.
Acknowledgments
We thank Dr. R. L. Post for invaluable comments on the manuscript. This work was supported by a grant (15/00-1) from the Israel Science Foundation.
Abbreviations
- AMP-PNP
5′-adenylyl-β,γ-imidodiphosphate
- Desferal
desferrioxamine mesylate
- P domain
phosphorylation domain
- N domain
nucleotide domain
- A domain
actuator or anchor domain
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.220332897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.220332897
References
- 1.Glynn I M. In: Enzymes of Biological Membranes. Martonosi A, editor. New York: Plenum; 1985. pp. 35–114. [Google Scholar]
- 2.Glynn I M, Karlish S J D. Annu Rev Biochem. 1990;59:171–205. doi: 10.1146/annurev.bi.59.070190.001131. [DOI] [PubMed] [Google Scholar]
- 3.Maclennan D H, Rice W J, Green N M. J Biol Chem. 1997;272:28815–28818. doi: 10.1074/jbc.272.46.28815. [DOI] [PubMed] [Google Scholar]
- 4.Toyoshima C, Nakasako M, Nomura H, Ogawa H. Nature (London) 2000;405:647–655. doi: 10.1038/35015017. [DOI] [PubMed] [Google Scholar]
- 5.Møller J V, Juul B, Le Maire M. Biochim Biophys Acta. 1996;1286:1–51. doi: 10.1016/0304-4157(95)00017-8. [DOI] [PubMed] [Google Scholar]
- 6.Andersen J P, Vilsen B. FEBS Lett. 1995;359:101–106. doi: 10.1016/0014-5793(95)00019-6. [DOI] [PubMed] [Google Scholar]
- 7.Aravind L, Galperin M Y, Koonin E V. Trends Biochem Sci. 1998;23:127–129. doi: 10.1016/s0968-0004(98)01189-x. [DOI] [PubMed] [Google Scholar]
- 8.Ridder I S, Dijkstra B W. Biochem J. 1999;339:223–226. [PMC free article] [PubMed] [Google Scholar]
- 9.Ogawa H, Stokes D L, Sasabe H, Toyoshima C. Biophys J. 1998;75:41–52. doi: 10.1016/S0006-3495(98)77493-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldshleger R, Karlish S J D. Proc Natl Acad Sci USA. 1997;94:9596–9601. doi: 10.1073/pnas.94.18.9596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldshleger R, Karlish S J D. J Biol Chem. 1999;274:16213–16221. doi: 10.1074/jbc.274.23.16213. [DOI] [PubMed] [Google Scholar]
- 12.Karlish S J D. Acta Physiol Scand. 1998;163:89–98. [PubMed] [Google Scholar]
- 13.Bar Shimon M, Goldshleger R, Karlish S J D. J Biol Chem. 1998;273:34190–34195. doi: 10.1074/jbc.273.51.34190. [DOI] [PubMed] [Google Scholar]
- 14.Jørgensen P L. Methods Enzymol. 1988;156:29–43. doi: 10.1016/0076-6879(88)56005-6. [DOI] [PubMed] [Google Scholar]
- 15.Karlish S J D. J Bioenerg Biomembr. 1980;12:111–135. doi: 10.1007/BF00744678. [DOI] [PubMed] [Google Scholar]
- 16.Capasso J M, Hoving S, Tal D M, Goldshleger R, Karlish S J D. J Biol Chem. 1992;267:1150–1158. [PubMed] [Google Scholar]
- 17.Goldshleger R, Tal D M, Karlish S J D. Biochemistry. 1995;34:8668–8679. doi: 10.1021/bi00027a016. [DOI] [PubMed] [Google Scholar]
- 18.Floyd R A, Lewis C A. Biochemistry. 1983;22:2645–2649. doi: 10.1021/bi00280a008. [DOI] [PubMed] [Google Scholar]
- 19.Vile G F, Winterbourn C C, Sutton H C. Arch Biochem Biophys. 1987;259:616–626. doi: 10.1016/0003-9861(87)90528-5. [DOI] [PubMed] [Google Scholar]
- 20.Rush J D, Maskos Z, Koppenol W H. FEBS Lett. 1990;261:121–123. [Google Scholar]
- 21.Hegevary C, Post R L. J Biol Chem. 1971;246:5235–5240. [PubMed] [Google Scholar]
- 22.Farley R A, Tran C M, Carilli C T, Hawke D, Shively J E. J Biol Chem. 1984;259:9532–9535. [PubMed] [Google Scholar]
- 23.Rendi R, Uhr M L. Biochim Biophys Acta. 1964;89:520–531. doi: 10.1016/0926-6569(64)90078-1. [DOI] [PubMed] [Google Scholar]
- 24.Fukushima Y, Post R L. J Biol Chem. 1978;253:6853–6872. [PubMed] [Google Scholar]
- 25.Fahn S, Koval G J, Albers R W. J Biol Chem. 1966;241:1882–1889. [PubMed] [Google Scholar]
- 26.Richards D E. J Physiol. 1988;404:497–514. doi: 10.1113/jphysiol.1988.sp017302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campos M, Beauge L. Biochim Biophys Acta. 1988;944:242–248. doi: 10.1016/0005-2736(88)90437-3. [DOI] [PubMed] [Google Scholar]
- 28.Post R L, Toda G, Rogers F N. J Biol Chem. 1975;250:691–701. [PubMed] [Google Scholar]
- 29.Grisham C. Methods Enzymol. 1988;156:353–370. doi: 10.1016/0076-6879(88)56036-6. [DOI] [PubMed] [Google Scholar]
- 30.Kasho V N, Stengelin M, Smirnova I N, Faller L D. Biochemistry. 1997;16:8045–8052. doi: 10.1021/bi970472z. [DOI] [PubMed] [Google Scholar]
- 31.Esmann M, Skou J C. Biochim Biophys Res Commun. 1985;127:857–863. doi: 10.1016/s0006-291x(85)80022-x. [DOI] [PubMed] [Google Scholar]
- 32.Webb M R, Trentham D R. J Biol Chem. 1981;256:4884–4887. [PubMed] [Google Scholar]
- 33.Repke K R H, Schön R. Biochim Biophys Acta. 1992;1154:1–16. doi: 10.1016/0304-4157(93)90014-f. [DOI] [PubMed] [Google Scholar]
- 34.De Meis L, Martins O B, Alves E W. Biochemistry. 1980;19:4252–4261. doi: 10.1021/bi00559a017. [DOI] [PubMed] [Google Scholar]
- 35.Schneeberger A, Apell H J. J Membr Biol. 1999;168:221–228. doi: 10.1007/s002329900511. [DOI] [PubMed] [Google Scholar]
- 36.Ward D G, Cavieres J D. J Biol Chem. 1996;271:12317–12321. doi: 10.1074/jbc.271.21.12317. [DOI] [PubMed] [Google Scholar]
- 37.Thoenges D, Schoner W. J Biol Chem. 1997;272:16315–16321. doi: 10.1074/jbc.272.26.16315. [DOI] [PubMed] [Google Scholar]