Abstract
BACKGROUND/AIM—Matrilysin is one of the matrix metalloproteinases that has a critical role in tumour invasion, and is often expressed in gastrointestinal cancers. The aim of this study was to examine the role of matrilysin in metastasis of human colorectal cancers. PATIENTS (SUBJECTS)/METHODS—The relation between matrilysin expression and Dukes's type was investigated immunohistochemically in 83 surgically resected colorectal cancers, including five with liver metastasis. Moreover, the effects of matrilysin on the in vivo invasive and metastatic potential of colon cancer cells transfected with matrilysin cDNA were examined after subcutaneous injection into SCID mice. RESULTS—In 46% of primary and all of metastatic liver tumours, over 10% of cancer cells were stained positively for matrilysin. The expression of matrilysin correlated significantly with the presence of nodal or distant metastases (p<0.05). In addition, matrilysin transfectants formed invasive tumours and multiple liver metastases in SCID mice, without producing any significant difference in the subcutaneous tumour growth from mock transfectants. Casein zymography showed that the invading and metastasised tumours showed conspicuous matrilysin activity, which correlated with the number of metastatic lesions (p<0.001). CONCLUSIONS—Matrilysin showed a correlation with metastasis in a cohort of 83 colorectal cancer patients and marked metastatic potentiation in human colorectal cancer xenografts, indicating that it may play a critical role in the metastatic pathway of colorectal cancers. Keywords: matrix metalloproteinase; matrilysin; MMP-7; colorectal cancer; metastasis; transfection; human
Full Text
The Full Text of this article is available as a PDF (215.9 KB).
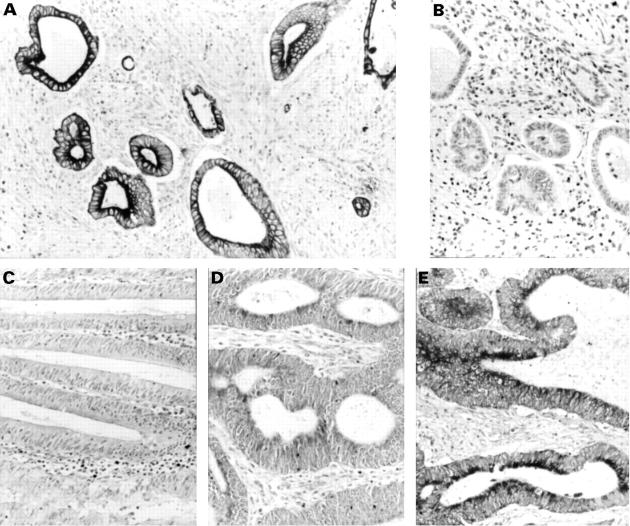
Figure 1 .
Immunostaining of human colon cancers with anti-matrilysin monoclonal antibody was performed as described in Materials and methods (original magnification × 200). (A) A well differentiated adenocarcinoma case of Dukes's type C. The monoclonal antibody stained tumour cell cytoplasm and cell membranes, but did not stain the stromal components. (B) Immunostaining with negative control monoclonal antibody of the serial section of (A). Adjacent normal colon epithelium (C) was not stained with anti-matrilysin monoclonal antibody. (D) A well differentiated adenocarcinoma case in Dukes's type D. Metastatic liver nests (E) were stained more strongly than the primary site (D).
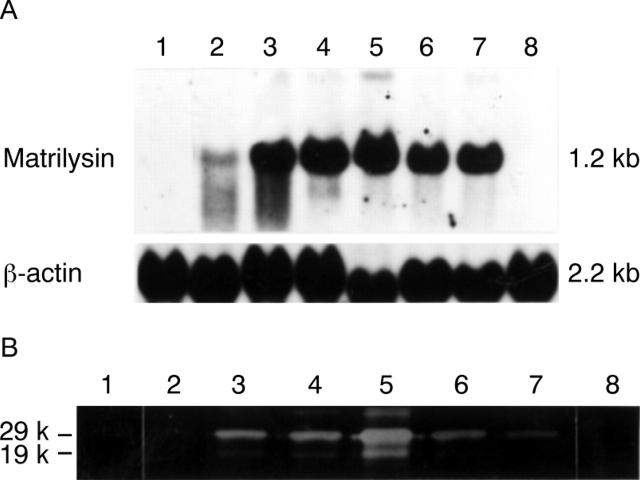
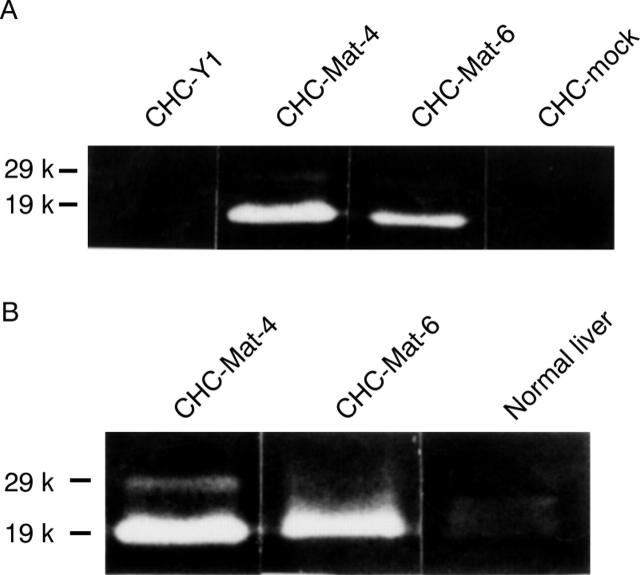
Figure 2 .
(A) Northern blot analysis and (B) casein zymography of parental cells, sublines of matrilysin transfectants, and mock transfectants were performed as described in Materials and methods. The matrilysin transfectants expressed various levels of matrilysin mRNA (A) and secreted promatrilysin (29 kDa) and activated matrilysin (19 kDa; B), but neither was produced by parental cells or mock transfectants: lane 1, CHC-Y1; lane 2, CHC-Mat-1; lane 3, CHC-Mat-2; lane 4, CHC-Mat-3; lane 5, CHC-Mat-4; lane 6, CHC-Mat-5; lane 7, CHC-Mat-6; lane 8, CHC-mock. Clones CHC-Mat-4 (lane 5) and CHC-Mat-6 (lane 7) were used as sublines expressing high and moderate levels of matrilysin respectively.
Figure 3 .
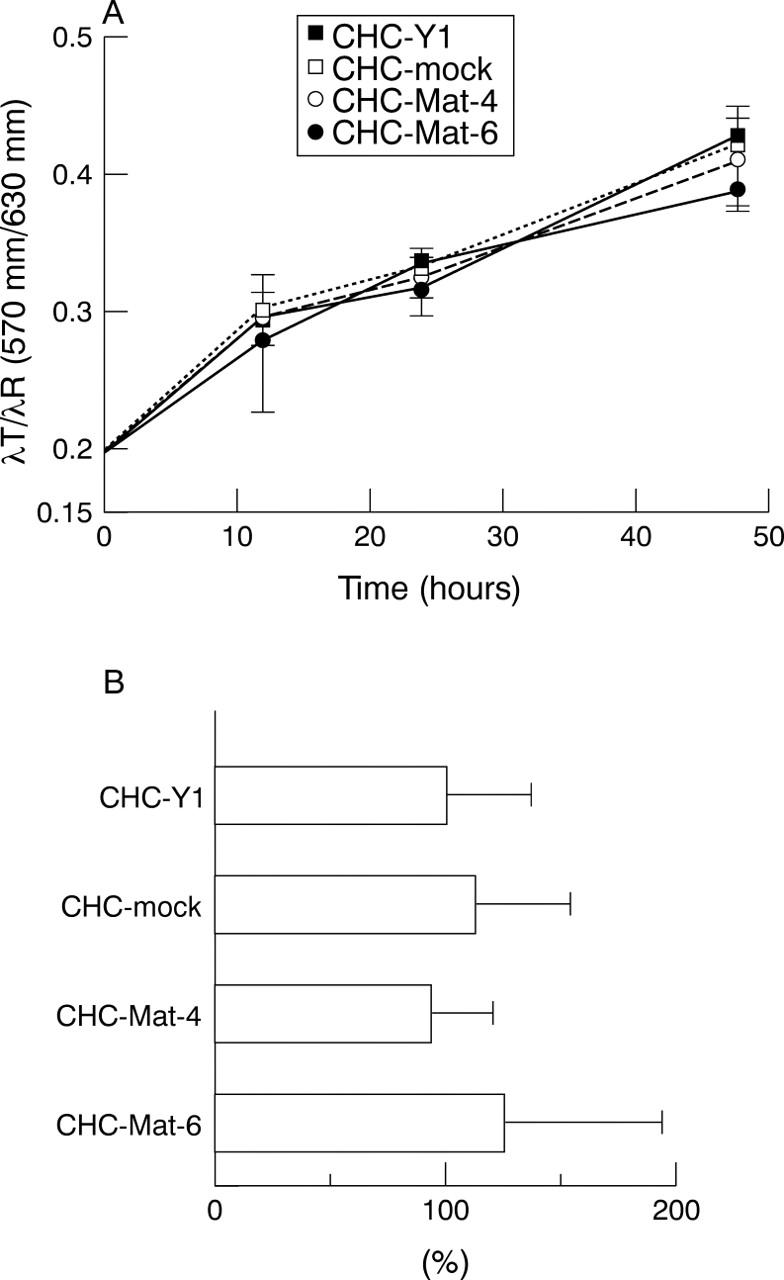
(A) 3-(4,5-Dimethylthiazol)-2,5-diphenyl- tetrazolium bromide assay showed that there was no significant difference in in vitro growth in CHC-Y1, mock transfectants, and matrilysin transfectants. (B) Gold colloid assay showed that in vitro motility of two matrilysin transfected sublines was equivalent to those of parental cell and mock transfectants.
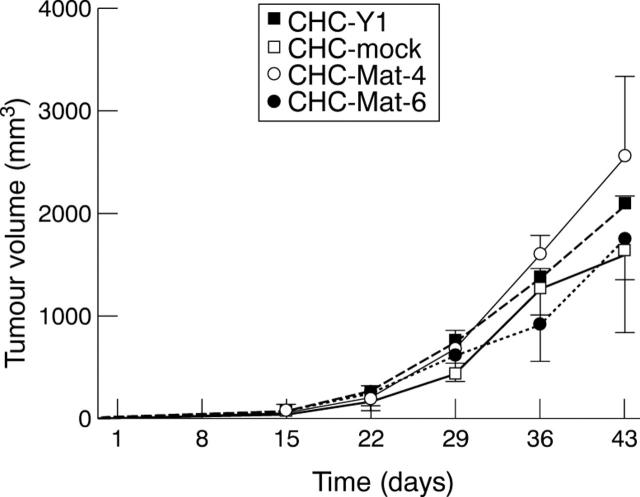
Figure 4 .
Tumour volumes of transplanted subcutaneous site in SCID mice were measured and calculated every seven days. Growth of tumours derived from two matrilysin transfected sublines was not significantly different from those derived from mock transfectants or parental cell CHC-Y1.
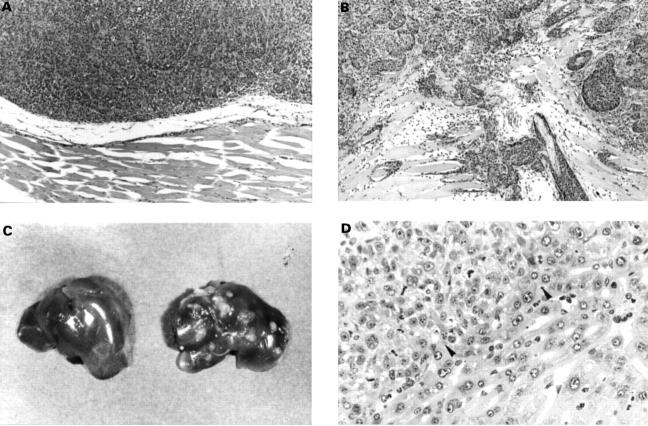
Figure 5 .
Each subline of matrilysin transfected cancer cells and mock transfectants formed almost the same sized subcutaneous tumour six weeks after injection into SCID mice. (A) Primary subcutaneous tumours of mock transfectants did not invade the muscle tissues (original magnification × 100). (B) Primary subcutaneous tumours of matrilysin transfectants CHC-Mat-6 invaded the muscle layer (original magnification × 100). (C) The mice injected with matrilysin transfectants showed multiple liver metastases (right), while those injected with mock transfectants did not (left). (D) Microscopic view of liver metastatic nest (C, right) showing colon cancer cells in left upper side of the field (original magnification × 400). Arrowheads indicate the border of tumour cells and hepatocytes.
Figure 6 .
Primary and metastatic tumours of SCID mice were analysed by zymography as described in Materials and methods. (A) Casein zymography showed that primary subcutaneous tumours derived from the two matrilysin transfectants produced matrilysin, the active form (19k) of which was much more abundant than the latent form (29k). (B) Casein zymography of liver showed that activated matrilysin was effectively expressed in metastatic lesions but neither form of matrilysin was detected in normal murine liver.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht-Buehler G. The phagokinetic tracks of 3T3 cells. Cell. 1977 Jun;11(2):395–404. doi: 10.1016/0092-8674(77)90057-5. [DOI] [PubMed] [Google Scholar]
- Baragi V. M., Fliszar C. J., Conroy M. C., Ye Q. Z., Shipley J. M., Welgus H. G. Contribution of the C-terminal domain of metalloproteinases to binding by tissue inhibitor of metalloproteinases. C-terminal truncated stromelysin and matrilysin exhibit equally compromised binding affinities as compared to full-length stromelysin. J Biol Chem. 1994 Apr 29;269(17):12692–12697. [PubMed] [Google Scholar]
- Birkedal-Hansen H., Moore W. G., Bodden M. K., Windsor L. J., Birkedal-Hansen B., DeCarlo A., Engler J. A. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Borchers A. H., Powell M. B., Fusenig N. E., Bowden G. T. Paracrine factor and cell-cell contact-mediated induction of protease and c-ets gene expression in malignant keratinocyte/dermal fibroblast cocultures. Exp Cell Res. 1994 Jul;213(1):143–147. doi: 10.1006/excr.1994.1183. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Contegiacomo A., Pizzi C., De Marchis L., Alimandi M., Delrio P., Di Palma E., Petrella G., Ottini L., French D., Frati L. High cell kinetics is associated with amplification of the int-2, bcl-1, myc and erbB-2 proto-oncogenes and loss of heterozygosity at the DF3 locus in primary breast cancers. Int J Cancer. 1995 Mar 29;61(1):1–6. doi: 10.1002/ijc.2910610102. [DOI] [PubMed] [Google Scholar]
- Crabbe T., Smith B., O'Connell J., Docherty A. Human progelatinase A can be activated by matrilysin. FEBS Lett. 1994 May 23;345(1):14–16. doi: 10.1016/0014-5793(94)00412-9. [DOI] [PubMed] [Google Scholar]
- Crawford H. C., Matrisian L. M. Mechanisms controlling the transcription of matrix metalloproteinase genes in normal and neoplastic cells. Enzyme Protein. 1996;49(1-3):20–37. doi: 10.1159/000468614. [DOI] [PubMed] [Google Scholar]
- DeClerck Y. A., Perez N., Shimada H., Boone T. C., Langley K. E., Taylor S. M. Inhibition of invasion and metastasis in cells transfected with an inhibitor of metalloproteinases. Cancer Res. 1992 Feb 1;52(3):701–708. [PubMed] [Google Scholar]
- Imai K., Yokohama Y., Nakanishi I., Ohuchi E., Fujii Y., Nakai N., Okada Y. Matrix metalloproteinase 7 (matrilysin) from human rectal carcinoma cells. Activation of the precursor, interaction with other matrix metalloproteinases and enzymic properties. J Biol Chem. 1995 Mar 24;270(12):6691–6697. doi: 10.1074/jbc.270.12.6691. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Ichikawa Y., Mitsuhashi M., Momiyama N., Chishima T., Tanaka K., Yamaoka H., Miyazakic K., Nagashima Y., Akitaya T. Matrilysin is associated with progression of colorectal tumor. Cancer Lett. 1996 Oct 1;107(1):5–10. doi: 10.1016/0304-3835(96)04336-4. [DOI] [PubMed] [Google Scholar]
- Itoh F., Yamamoto H., Hinoda Y., Imai K. Enhanced secretion and activation of matrilysin during malignant conversion of human colorectal epithelium and its relationship with invasive potential of colon cancer cells. Cancer. 1996 Apr 15;77(8 Suppl):1717–1721. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1717::AID-CNCR45>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Levy A. T., Cioce V., Sobel M. E., Garbisa S., Grigioni W. F., Liotta L. A., Stetler-Stevenson W. G. Increased expression of the Mr 72,000 type IV collagenase in human colonic adenocarcinoma. Cancer Res. 1991 Jan 1;51(1):439–444. [PubMed] [Google Scholar]
- Liabakk N. B., Talbot I., Smith R. A., Wilkinson K., Balkwill F. Matrix metalloprotease 2 (MMP-2) and matrix metalloprotease 9 (MMP-9) type IV collagenases in colorectal cancer. Cancer Res. 1996 Jan 1;56(1):190–196. [PubMed] [Google Scholar]
- Marks J. W., Uhler M. L., Bonorris G. G., Judd H. L. Nucleation of biliary cholesterol, arachidonate, prostaglandin E2, and glycoproteins in postmenopausal women. Gastroenterology. 1997 Apr;112(4):1271–1276. doi: 10.1016/s0016-5085(97)70140-9. [DOI] [PubMed] [Google Scholar]
- McDonnell S., Navre M., Coffey R. J., Jr, Matrisian L. M. Expression and localization of the matrix metalloproteinase pump-1 (MMP-7) in human gastric and colon carcinomas. Mol Carcinog. 1991;4(6):527–533. doi: 10.1002/mc.2940040617. [DOI] [PubMed] [Google Scholar]
- Miyazaki K., Hattori Y., Umenishi F., Yasumitsu H., Umeda M. Purification and characterization of extracellular matrix-degrading metalloproteinase, matrin (pump-1), secreted from human rectal carcinoma cell line. Cancer Res. 1990 Dec 15;50(24):7758–7764. [PubMed] [Google Scholar]
- Mori M., Barnard G. F., Mimori K., Ueo H., Akiyoshi T., Sugimachi K. Overexpression of matrix metalloproteinase-7 mRNA in human colon carcinomas. Cancer. 1995 Mar 15;75(6 Suppl):1516–1519. doi: 10.1002/1097-0142(19950315)75:6+<1516::aid-cncr2820751522>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Murray G. I., Duncan M. E., O'Neil P., Melvin W. T., Fothergill J. E. Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat Med. 1996 Apr;2(4):461–462. doi: 10.1038/nm0496–461. [DOI] [PubMed] [Google Scholar]
- Nakajima M., Morikawa K., Fabra A., Bucana C. D., Fidler I. J. Influence of organ environment on extracellular matrix degradative activity and metastasis of human colon carcinoma cells. J Natl Cancer Inst. 1990 Dec 19;82(24):1890–1898. doi: 10.1093/jnci/82.24.1890. [DOI] [PubMed] [Google Scholar]
- Newell K. J., Witty J. P., Rodgers W. H., Matrisian L. M. Expression and localization of matrix-degrading metalloproteinases during colorectal tumorigenesis. Mol Carcinog. 1994 Aug;10(4):199–206. doi: 10.1002/mc.2940100404. [DOI] [PubMed] [Google Scholar]
- Ohuchi E., Azumano I., Yoshida S., Iwata K., Okada Y. A one-step sandwich enzyme immunoassay for human matrix metalloproteinase 7 (matrilysin) using monoclonal antibodies. Clin Chim Acta. 1996 Jan 31;244(2):181–198. doi: 10.1016/0009-8981(95)06199-1. [DOI] [PubMed] [Google Scholar]
- Okada A., Bellocq J. P., Rouyer N., Chenard M. P., Rio M. C., Chambon P., Basset P. Membrane-type matrix metalloproteinase (MT-MMP) gene is expressed in stromal cells of human colon, breast, and head and neck carcinomas. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2730–2734. doi: 10.1073/pnas.92.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte H., Chastre E., Prevot S., Nordlinger B., Empereur S., Basset P., Chambon P., Gespach C. Neoplastic progression of human colorectal cancer is associated with overexpression of the stromelysin-3 and BM-40/SPARC genes. Int J Cancer. 1995 Feb 20;64(1):70–75. doi: 10.1002/ijc.2910640114. [DOI] [PubMed] [Google Scholar]
- Powell W. C., Knox J. D., Navre M., Grogan T. M., Kittelson J., Nagle R. B., Bowden G. T. Expression of the metalloproteinase matrilysin in DU-145 cells increases their invasive potential in severe combined immunodeficient mice. Cancer Res. 1993 Jan 15;53(2):417–422. [PubMed] [Google Scholar]
- Pyke C., Ralfkiaer E., Tryggvason K., Danø K. Messenger RNA for two type IV collagenases is located in stromal cells in human colon cancer. Am J Pathol. 1993 Feb;142(2):359–365. [PMC free article] [PubMed] [Google Scholar]
- Sato H., Takino T., Okada Y., Cao J., Shinagawa A., Yamamoto E., Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994 Jul 7;370(6484):61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- Sato Y., Mukai K., Watanabe S., Goto M., Shimosato Y. The AMeX method. A simplified technique of tissue processing and paraffin embedding with improved preservation of antigens for immunostaining. Am J Pathol. 1986 Dec;125(3):431–435. [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Hewitt R., Corcoran M. Matrix metalloproteinases and tumor invasion: from correlation and causality to the clinic. Semin Cancer Biol. 1996 Jun;7(3):147–154. doi: 10.1006/scbi.1996.0020. [DOI] [PubMed] [Google Scholar]
- Wilson C. L., Matrisian L. M. Matrilysin: an epithelial matrix metalloproteinase with potentially novel functions. Int J Biochem Cell Biol. 1996 Feb;28(2):123–136. doi: 10.1016/1357-2725(95)00121-2. [DOI] [PubMed] [Google Scholar]
- Witty J. P., McDonnell S., Newell K. J., Cannon P., Navre M., Tressler R. J., Matrisian L. M. Modulation of matrilysin levels in colon carcinoma cell lines affects tumorigenicity in vivo. Cancer Res. 1994 Sep 1;54(17):4805–4812. [PubMed] [Google Scholar]
- Woessner J. F., Jr, Taplin C. J. Purification and properties of a small latent matrix metalloproteinase of the rat uterus. J Biol Chem. 1988 Nov 15;263(32):16918–16925. [PubMed] [Google Scholar]
- Yamamoto H., Itoh F., Hinoda Y., Senota A., Yoshimoto M., Nakamura H., Imai K., Yachi A. Expression of matrilysin mRNA in colorectal adenomas and its induction by truncated fibronectin. Biochem Biophys Res Commun. 1994 Jun 15;201(2):657–664. doi: 10.1006/bbrc.1994.1751. [DOI] [PubMed] [Google Scholar]
- Yoshimoto M., Itoh F., Yamamoto H., Hinoda Y., Imai K., Yachi A. Expression of MMP-7(PUMP-1) mRNA in human colorectal cancers. Int J Cancer. 1993 Jun 19;54(4):614–618. doi: 10.1002/ijc.2910540415. [DOI] [PubMed] [Google Scholar]
- Zeng Z. S., Guillem J. G. Distinct pattern of matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 mRNA expression in human colorectal cancer and liver metastases. Br J Cancer. 1995 Sep;72(3):575–582. doi: 10.1038/bjc.1995.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker S., Lysik R. M., DiMassimo B. I., Zarrabi H. M., Moll U. M., Grimson R., Tickle S. P., Docherty A. J. Plasma assay of gelatinase B: tissue inhibitor of metalloproteinase complexes in cancer. Cancer. 1995 Aug 15;76(4):700–708. doi: 10.1002/1097-0142(19950815)76:4<700::aid-cncr2820760426>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]