Abstract
BACKGROUND—Inactivity of the gut leads to atrophic changes of which little is known. AIMS—To investigate structural, neuronal, and functional changes occurring in bypassed rat ileum. METHODS—Morphometry was used to characterise the atrophic changes. The numbers of enteric neurones, their expression of neurotransmitters, and the presence of interstitial cells of Cajal were studied using immunocytochemistry and in situ hybridisation. Motor activity was studied in vitro. RESULTS—Adaptive changes in bypassed ileum include atrophy and remodelling of the gut wall. The total numbers of submucous and myenteric neurones per unit length increased one and four weeks after bypass but were identical to sham operated intestine 10 weeks after bypass. Neurones expressing vasoactive intestinal peptide, neuropeptide Y, or pituitary adenylate cyclase activating peptide decreased gradually in number in bypassed ileum. Nitric oxide synthase expressing neurones were increased, particularly in the myenteric ganglia. No change in the frequency and distribution of interstitial cells of Cajal was noted. The contractile response elicited by electrical stimulation of sham operated ileum consisted of a fast cholinergic twitch followed by a slower non-adrenergic, non-cholinergic contraction. In the bypassed ileum an identical biphasic contraction was elicited; however, the entire response was non-adrenergic, non-cholinergic. The relaxatory response to electrical stimulation in sham operated ileum was nitric oxide mediated; after bypass it was non-nitrergic. CONCLUSIONS—Notable atrophic changes were seen in the rat ileum after bypass. The enteric nervous system reacted with neuronal cell death and plasticity in terms of release and expression of neurotransmitters. Keywords: neuronal plasticity; enteric nerves; interstitial cells of Cajal; atrophy; neuropeptides; nitric oxide
Full Text
The Full Text of this article is available as a PDF (256.9 KB).
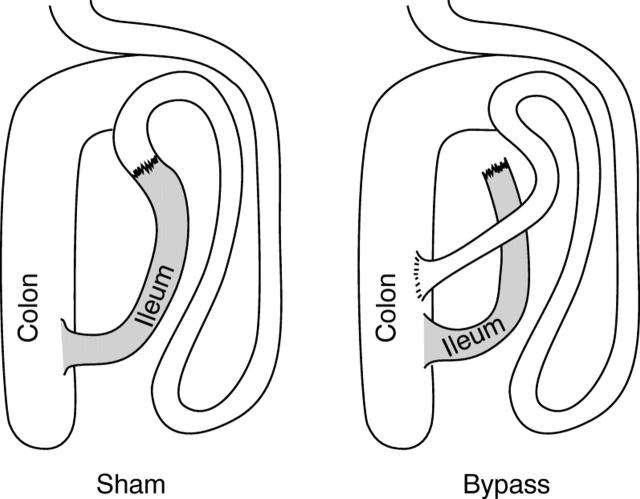
Figure 1 .
Schematic outline of the surgical procedures used.
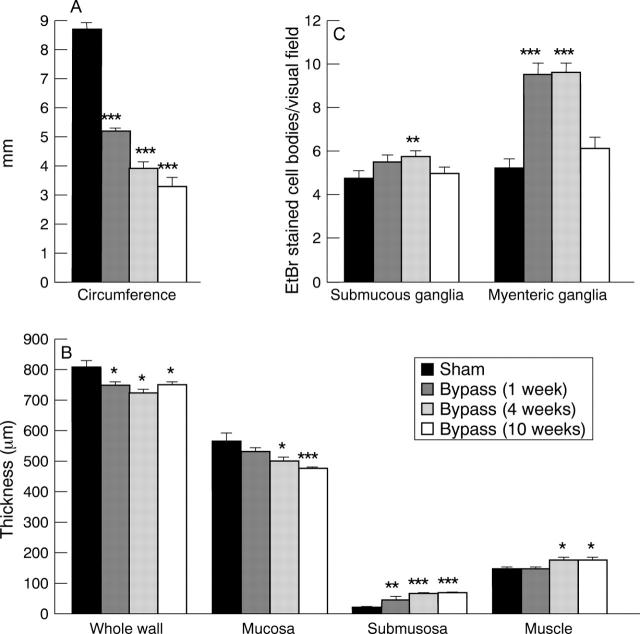
Figure 2 .
Measurements on the circumference (A) and thickness of the whole wall, mucosa, submucosa, and muscle layers (B) in rat ileum, sham operated and bypassed for one, four, or 10 weeks. (C) Total numbers of submucous and myenteric nerve cell bodies (as estimated by EtBr staining) per visual field in longitudinally cut sections from sham operated and bypassed rats. Mean (SEM). *p<0.05, **p<0.01, ***p<0.001 compared with sham operated.
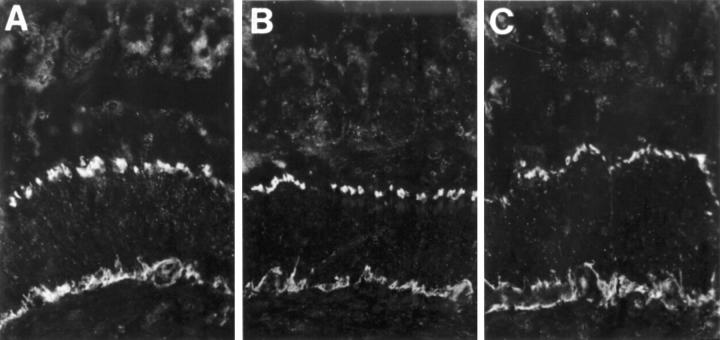
Figure 3 .
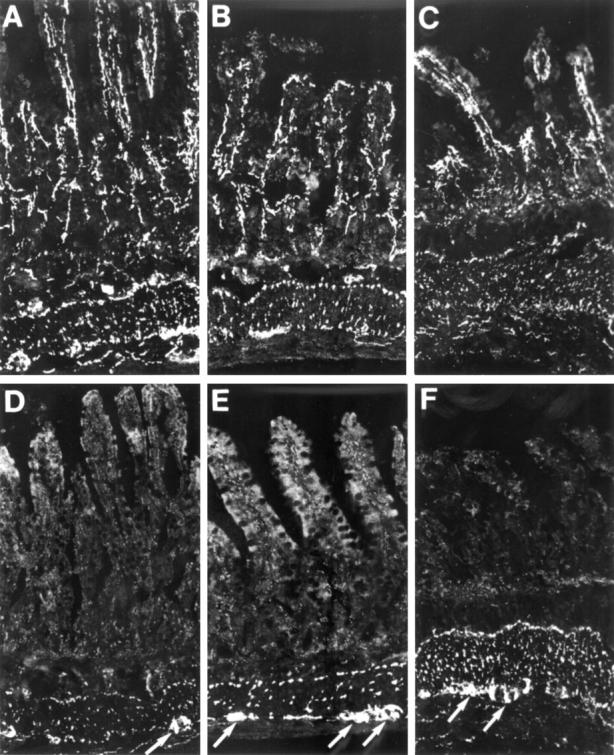
Cryostat sections from rat, sham operated (A and D), one week (B and E), and 10 weeks (C and F) bypassed ileum, immunostained for VIP (A-C) and nNOS (D-F). VIP immunoreactive nerve fibres are numerous in mucosa/submucosa, muscular layers, and enteric ganglia at all time points studied. nNOS immunoreactive nerve fibres are restricted to the muscle layers and myenteric ganglia. The number of nNOS immunoreactive cell bodies within myenteric ganglia (indicated by arrows) is increased in bypassed ileum, particularly 10 weeks postoperatively (F). Original magnification × 130.
Figure 4 .
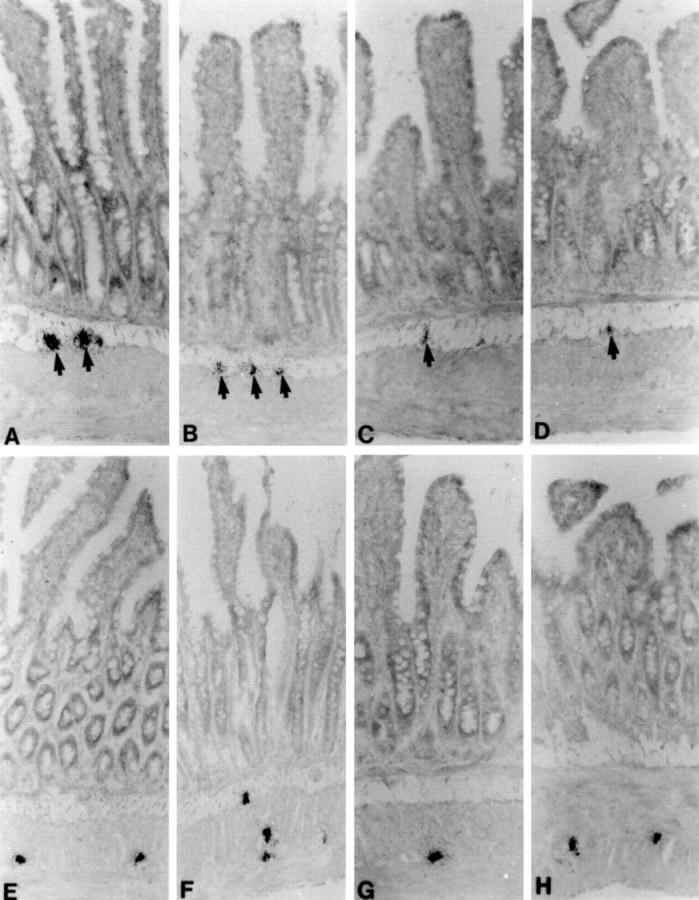
Cryostat sections from rat, sham operated (A and E), one week (B and F), four weeks (C and G), and 10 weeks (D and H) bypassed ileum autoradiographically labelled for VIP mRNA (A-D) and galanin mRNA (E-H). VIP mRNA containing submucous neurones (indicated by arrows) are intensely labelled in sham operated ileum (A) but only weakly labelled in the bypassed ileum (B-D). The labelling of galanin mRNA containing enteric neurones is equally intense in both sham operated (E) and bypassed (F-H) ileum. Original magnification × 130.
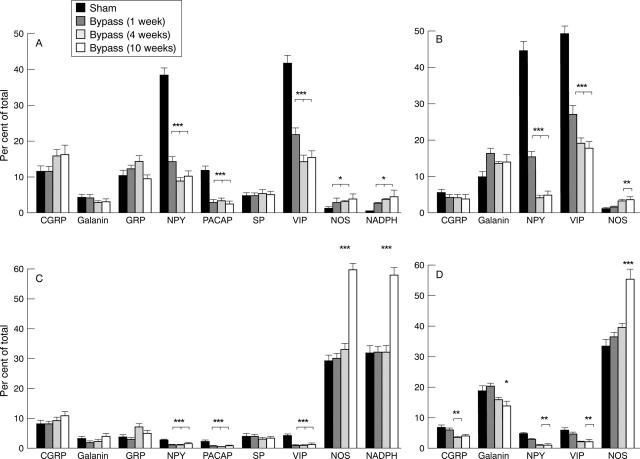
Figure 5 .
Numbers of CGRP, galanin, GRP, NPY, PACAP, SP, VIP, and nNOS immunoreactive, NADPH diaphorase positive (A and C); and CGRP mRNA, galanin mRNA, NPY mRNA, VIP mRNA, and nNOS mRNA expressing (B and D) neuronal cell bodies in submucous (A and B) and myenteric (C and D) ganglia from sham operated and bypassed (1-10 weeks) rat ileum. The numbers of positive cell bodies are expressed as the percentage of the total number of cell bodies, established by EtBr staining. Mean (SEM). *p<0.05, **p<0.01, ***p<0.001 compared with sham operated; p values refer to one, two, or three bars, as indicated.
Figure 6 .
Cryostat sections from rat: (A) sham operated, (B) one week, and (C) 10 weeks bypassed ileum, showing the presence of ICC using antiserum against c-kit receptor. Numerous ICC are found within the deep muscular plexus and at the border of longitudinal and circular muscle in both sham operated and bypassed ileum. Original magnification × 180.
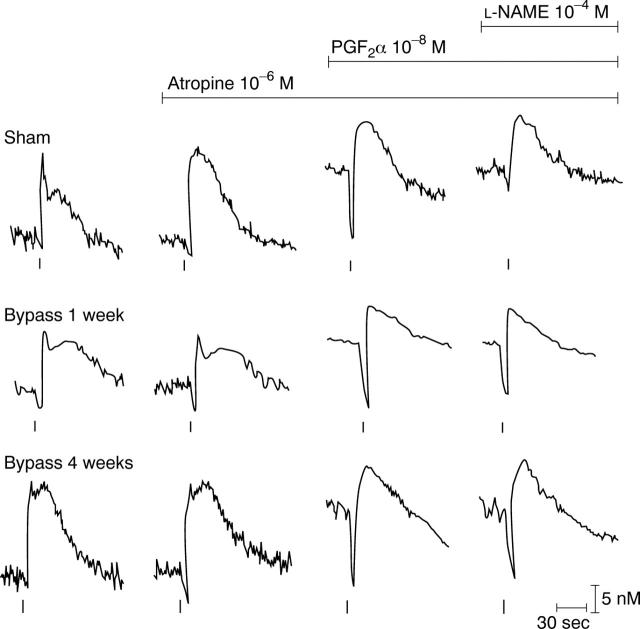
Figure 7 .
Tracings of electrically induced responses of rat sham operated, one week, and four weeks bypassed ileum. Stimulation (20 Hz, 4 V, 400 mA) was maintained for five seconds (indicated by vertical bars). The contractile response elicited by electrical stimulation of sham operated ileum consists of a fast cholinergic twitch followed by a slower non-adrenergic, non-cholinergic (NANC) mediated contraction; in the bypassed ileum an identical biphasic contraction is elicited; however, the entire response was NANC mediated—that is, not blocked by atropine. After precontraction by PGF2α and in the presence of atropine a relaxation followed by a contraction is elicited by EFS. The EFS induced relaxatory response in sham operated ileum is abolished by L-NAME and thus, NO mediated. After bypass the relaxation was unaffected by pretreatment with L-NAME.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belai A., Boulos P. B., Robson T., Burnstock G. Neurochemical coding in the small intestine of patients with Crohn's disease. Gut. 1997 Jun;40(6):767–774. doi: 10.1136/gut.40.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belai A., Cooper S., Burnstock G. Effect of age on NADPH-diaphorase-containing myenteric neurones of rat ileum and proximal colon. Cell Tissue Res. 1995 Feb;279(2):379–383. doi: 10.1007/BF00318495. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Brenneman D. E., Eiden L. E. Vasoactive intestinal peptide and electrical activity influence neuronal survival. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1159–1162. doi: 10.1073/pnas.83.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A. L., Moukarzel A. A., Ament M. E., Eckhert C., Bhuta S., Mestecky J., Hollander D. Effects of total parenteral nutrition on intestinal morphology and function in humans. Transplant Proc. 1994 Jun;26(3):1457–1457. [PubMed] [Google Scholar]
- Chin B. C., Tan D. T., Scott R. B. Depressed smooth muscle contractility after massive intestinal resection in rat: role of alterations in muscarinic receptor status or source of calcium for excitation-contraction coupling. Can J Physiol Pharmacol. 1996 Nov;74(11):1187–1195. doi: 10.1139/cjpp-74-11-1187. [DOI] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M. Nitric oxide neurotoxicity. J Chem Neuroanat. 1996 Jun;10(3-4):179–190. doi: 10.1016/0891-0618(96)00148-2. [DOI] [PubMed] [Google Scholar]
- Dowling R. H. Cellular and molecular basis of intestinal and pancreatic adaptation. Scand J Gastroenterol Suppl. 1992;193:64–67. doi: 10.3109/00365529209096008. [DOI] [PubMed] [Google Scholar]
- Ekblad E., Alm P., Sundler F. Distribution, origin and projections of nitric oxide synthase-containing neurons in gut and pancreas. Neuroscience. 1994 Nov;63(1):233–248. doi: 10.1016/0306-4522(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Ekblad E., Ekelund M., Sundler F. Relaxant responses of VIP and PACAP in rat ileum: receptors and adaptive supersensitivity. Ann N Y Acad Sci. 1998 Dec 11;865:393–396. doi: 10.1111/j.1749-6632.1998.tb11203.x. [DOI] [PubMed] [Google Scholar]
- Ekblad E., Ekman R., Håkanson R., Sundler F. Projections of peptide-containing neurons in rat colon. Neuroscience. 1988 Nov;27(2):655–674. doi: 10.1016/0306-4522(88)90296-5. [DOI] [PubMed] [Google Scholar]
- Ekblad E., Mulder H., Sundler F. Vasoactive intestinal peptide expression in enteric neurons is upregulated by both colchicine and axotomy. Regul Pept. 1996 Jul 5;63(2-3):113–121. doi: 10.1016/0167-0115(96)00028-6. [DOI] [PubMed] [Google Scholar]
- Ekblad E., Rökaeus A., Håkanson R., Sundler F. Galanin nerve fibers in the rat gut: distribution, origin and projections. Neuroscience. 1985 Oct;16(2):355–363. doi: 10.1016/0306-4522(85)90008-9. [DOI] [PubMed] [Google Scholar]
- Ekblad E., Sjuve R., Arner A., Sundler F. Enteric neuronal plasticity and a reduced number of interstitial cells of Cajal in hypertrophic rat ileum. Gut. 1998 Jun;42(6):836–844. doi: 10.1136/gut.42.6.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblad E., Sundler F. Motor responses in rat ileum evoked by nitric oxide donors vs. field stimulation: modulation by pituitary adenylate cyclase-activating peptide, forskolin and guanylate cyclase inhibitors. J Pharmacol Exp Ther. 1997 Oct;283(1):23–28. [PubMed] [Google Scholar]
- Ekblad E., Winther C., Ekman R., Håkanson R., Sundler F. Projections of peptide-containing neurons in rat small intestine. Neuroscience. 1987 Jan;20(1):169–188. doi: 10.1016/0306-4522(87)90010-8. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Young H. M., Pompolo S., Bornstein J. C., Kunze W. A., McConalogue K. Plurichemical transmission and chemical coding of neurons in the digestive tract. Gastroenterology. 1995 Feb;108(2):554–563. doi: 10.1016/0016-5085(95)90086-1. [DOI] [PubMed] [Google Scholar]
- Gabella G. Hypertrophy of intestinal smooth muscle. Cell Tissue Res. 1975 Nov 7;163(2):199–214. [PubMed] [Google Scholar]
- Gabella G. Hypertrophy of visceral smooth muscle. Anat Embryol (Berl) 1990;182(5):409–424. doi: 10.1007/BF00178906. [DOI] [PubMed] [Google Scholar]
- Hope B. T., Michael G. J., Knigge K. M., Vincent S. R. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen H. H., Wiklund N. P., Olgart C., Gustafsson L. E. Nerve stimulation-induced nitric oxide release as a consequence of muscarinic M1 receptor activation. Eur J Pharmacol. 1997 Jul 23;331(2-3):213–219. doi: 10.1016/s0014-2999(97)01027-3. [DOI] [PubMed] [Google Scholar]
- Jaffrey S. R., Snyder S. H. Nitric oxide: a neural messenger. Annu Rev Cell Dev Biol. 1995;11:417–440. doi: 10.1146/annurev.cb.11.110195.002221. [DOI] [PubMed] [Google Scholar]
- Jones B. A., Gores G. J. Physiology and pathophysiology of apoptosis in epithelial cells of the liver, pancreas, and intestine. Am J Physiol. 1997 Dec;273(6 Pt 1):G1174–G1188. doi: 10.1152/ajpgi.1997.273.6.G1174. [DOI] [PubMed] [Google Scholar]
- Kaplan L. M., Spindel E. R., Isselbacher K. J., Chin W. W. Tissue-specific expression of the rat galanin gene. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1065–1069. doi: 10.1073/pnas.85.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissmeyer-Nielsen P., Christensen H., Laurberg S. Diverting colostomy induces mucosal and muscular atrophy in rat distal colon. Gut. 1994 Sep;35(9):1275–1281. doi: 10.1136/gut.35.9.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larhammar D., Ericsson A., Persson H. Structure and expression of the rat neuropeptide Y gene. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2068–2072. doi: 10.1073/pnas.84.7.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Mulder H., Uddman R., Moller K., Zhang Y. Z., Ekblad E., Alumets J., Sundler F. Pituitary adenylate cyclase activating polypeptide expression in sensory neurons. Neuroscience. 1994 Nov;63(1):307–312. doi: 10.1016/0306-4522(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Nishizawa M., Hayakawa Y., Yanaihara N., Okamoto H. Nucleotide sequence divergence and functional constraint in VIP precursor mRNA evolution between human and rat. FEBS Lett. 1985 Apr 8;183(1):55–59. doi: 10.1016/0014-5793(85)80953-4. [DOI] [PubMed] [Google Scholar]
- Santer R. M., Baker D. M. Enteric neuron numbers and sizes in Auerbach's plexus in the small and large intestine of adult and aged rats. J Auton Nerv Syst. 1988 Nov;25(1):59–67. doi: 10.1016/0165-1838(88)90008-2. [DOI] [PubMed] [Google Scholar]
- Schmued L. C., Swanson L. W., Sawchenko P. E. Some fluorescent counterstains for neuroanatomical studies. J Histochem Cytochem. 1982 Feb;30(2):123–128. doi: 10.1177/30.2.6174560. [DOI] [PubMed] [Google Scholar]
- Soediono P., Burnstock G. Contribution of ATP and nitric oxide to NANC inhibitory transmission in rat pyloric sphincter. Br J Pharmacol. 1994 Nov;113(3):681–686. doi: 10.1111/j.1476-5381.1994.tb17046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundler F., Ekblad E., Absood A., Håkanson R., Köves K., Arimura A. Pituitary adenylate cyclase activating peptide: a novel vasoactive intestinal peptide-like neuropeptide in the gut. Neuroscience. 1992;46(2):439–454. doi: 10.1016/0306-4522(92)90064-9. [DOI] [PubMed] [Google Scholar]
- Tanaka J., Koshimura K., Murakami Y., Kato Y. Stimulatory effect of PACAP on neuronal cell survival. Ann N Y Acad Sci. 1996 Dec 26;805:473–475. doi: 10.1111/j.1749-6632.1996.tb17506.x. [DOI] [PubMed] [Google Scholar]
- Vanderhoof J. A., Langnas A. N. Short-bowel syndrome in children and adults. Gastroenterology. 1997 Nov;113(5):1767–1778. doi: 10.1053/gast.1997.v113.pm9352883. [DOI] [PubMed] [Google Scholar]
- Wolvekamp M. C., Heineman E., Taylor R. G., Fuller P. J. Towards understanding the process of intestinal adaptation. Dig Dis. 1996 Jan-Feb;14(1):59–72. doi: 10.1159/000171539. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Koch T. R., Mustin E., Walgenbach-Telford S., Telford G. L. Muscarinic cholinergic receptor density following small intestinal transplantation in rats. Am J Physiol. 1993 Dec;265(6 Pt 1):G1057–G1063. doi: 10.1152/ajpgi.1993.265.6.G1057. [DOI] [PubMed] [Google Scholar]