Abstract
BACKGROUND—Oxidative and nitrosative stress have been implicated in the pathogenesis of inflammatory bowel diseases. AIMS—To study the role of nitric oxide (NO) derived from inducible NO synthase (iNOS) in an experimental model of murine enterocolitis. METHODS—Trinitrobenzene sulphonic acid (TNBS) was instilled per rectum to induce a lethal colitis in iNOS deficient mice and in wild type controls. The distal colon was evaluated for histological evidence of inflammation, iNOS expression and activity, tyrosine nitration and malondialdehyde formation (as indexes of nitrosative and oxidative stress), myeloperoxidase activity (as index of neutrophil infiltration), and tissue localisation of intercellular adhesion molecule 1 (ICAM-1). RESULTS—TNBS administration induced a high mortality and weight loss associated with a severe colonic mucosal erosion and ulceration, increased myeloperoxidase activity, increased concentrations of malondialdehyde, and an intense staining for nitrotyrosine and ICAM-1 in wild type mice. Genetic ablation of iNOS gene conferred to mice a significant resistance to TNBS induced lethality and colonic damage, and notably reduced nitrotyrosine formation and concentrations of malondialdehyde; it did not, however, affect neutrophil infiltration and intestinal ICAM-1 expression in the injured tissue. CONCLUSION—Data show that activation of iNOS is required for nitrosative and oxidative damage in experimental colitis. Keywords: nitric oxide; nitric oxide synthase; inflammatory bowel disease; intercellular adhesion molecule 1; malondialdehyde; nitrotyrosine
Full Text
The Full Text of this article is available as a PDF (359.0 KB).
Figure 1 .
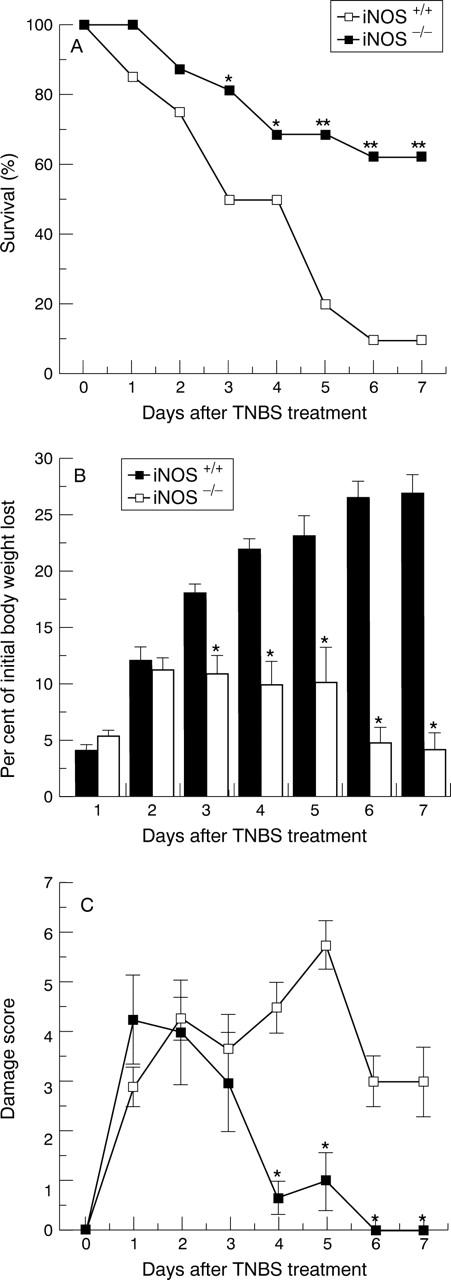
Survival (A), weight loss (B), and damage score (C) in iNOS+/+ and iNOS−/− mice after TNBS intracolonic administration. Each data point is the mean (SEM) of 8-20 animals for each group. *p<0.05, **p<0.01.
Figure 2 .
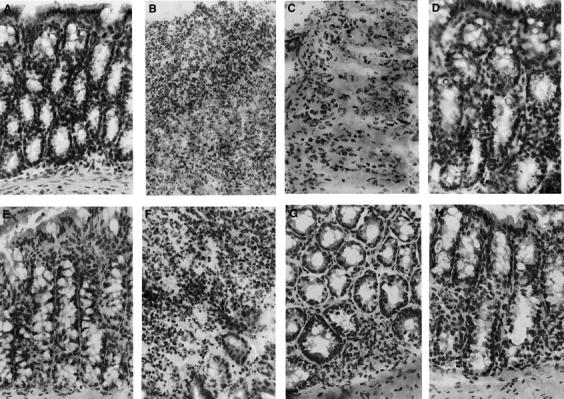
Time course of changes of colonic epithelial architecture after TNBS administration. Representative colonic sections from non-treated iNOS+/+ (A) or iNOS−/− (E) mice show normal tissue structure at day 0. (B) and (C) show a notable disruption of the epithelial structure with extensive haemorrhagic necrosis and infiltration of neutrophils in a colonic section from an iNOS+/+ mouse at two and four days after TNBS administration, respectively. At day 7 (D) oedema was still present in the healing epithelium of a TNBS treated iNOS+/+ mouse. (F) shows alteration of epithelial architecture with a massive infiltration of inflammatory cells in a colonic section of an iNOS−/− mouse two days after TNBS administration. At days 4 (G) and 7 (H) inflammatory cells were still present, mainly in the submucosa, while a healing process started in the epithelium. Original magnification × 400.
Figure 3 .
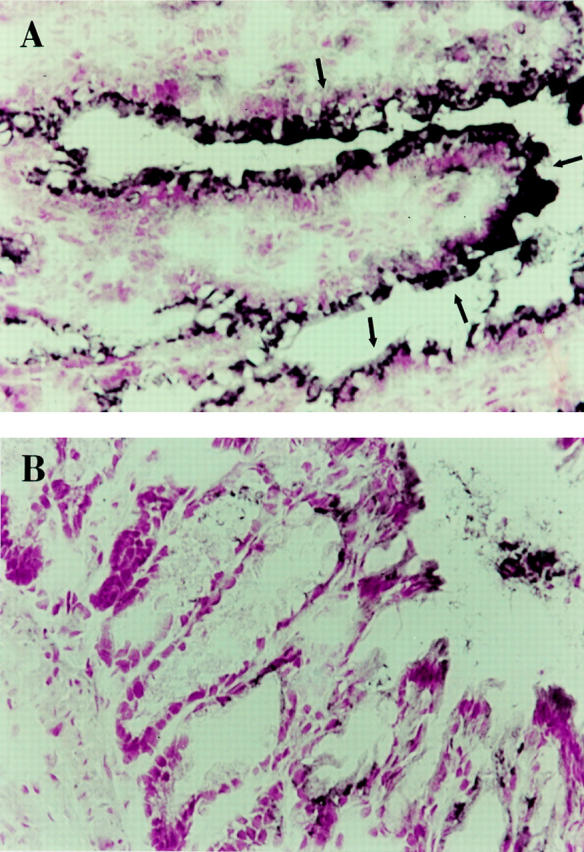
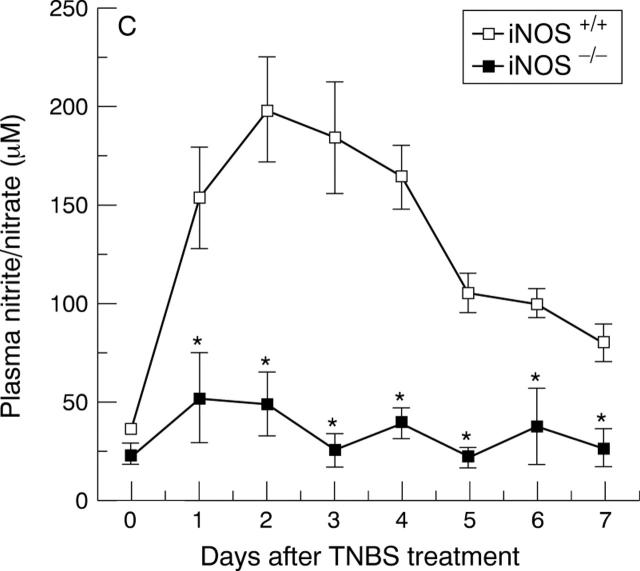
Immunohistochemical localisation of iNOS (A and B) and plasma nitrite/nitrate concentrations (C). (A) shows a diffuse dark staining localised in the apical epithelium (arrows) in the inflamed colon of an iNOS+/+ mouse four days after TNBS administration. (B) shows no staining in a colonic section of an iNOS−/− mouse four days after TNBS administration. Original magnification × 400. (C) shows the time course of plasma nitrite/nitrate concentrations after TNBS administration. Each data point is the mean (SEM) of 4-8 animals for each group. *p<0.05.
Figure 4 .
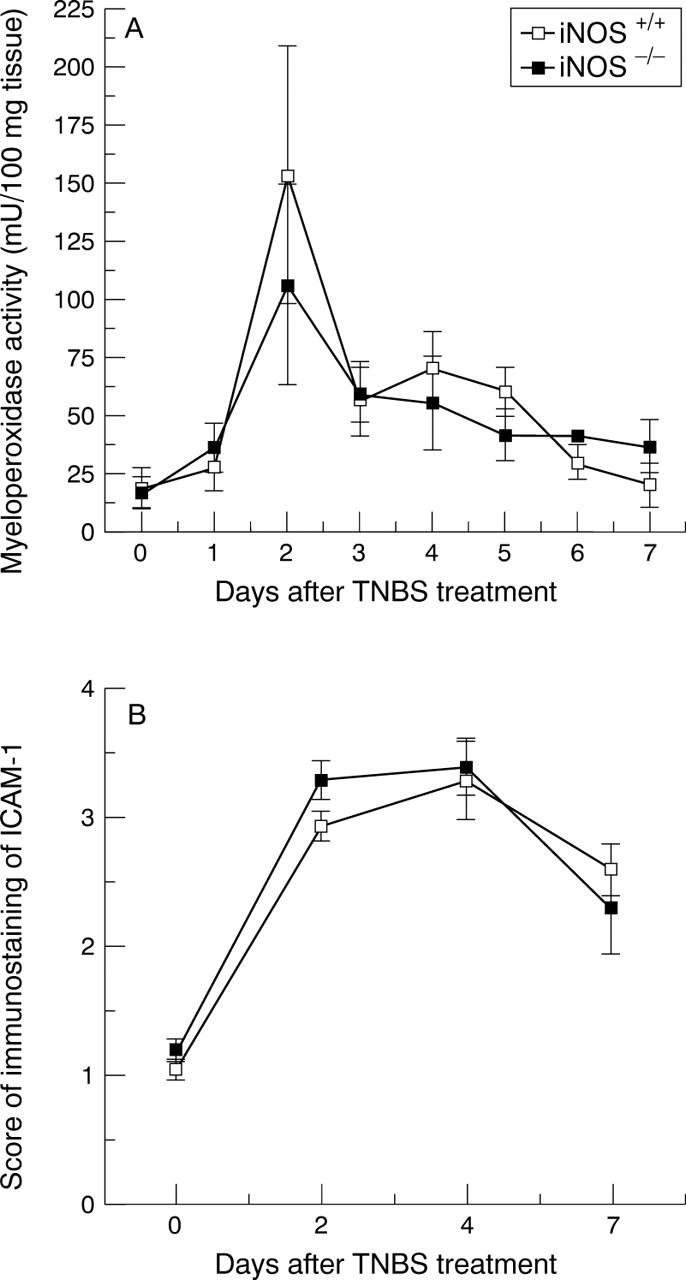
Time course of myeloperoxidase activity (A) and score of immunostaining of ICAM-1 (B) in tissues from iNOS+/+ and iNOS−/− mice after induction of colitis. Each data point is the mean (SEM) of eight animals for each group.
Figure 5 .
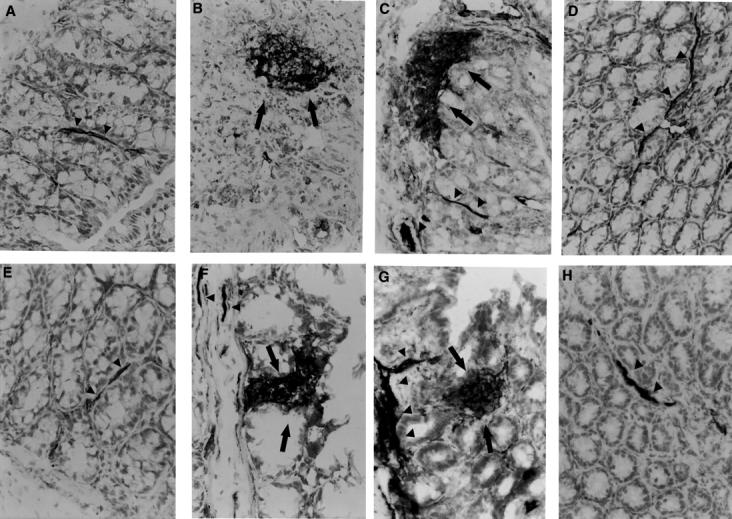
Time course of ICAM-1 expression after TNBS induced colitis. Control tissues at day 0 from non-treated animals iNOS+/+ (A) and iNOS-/- (E) showed a dark brown staining of endothelium of blood vessels (arrow heads) indicating the presence of constitutive ICAM-1 protein. TNBS administration induced an increase in the positive staining for ICAM-1 along the endothelial vascular wall (arrow heads) in the submucosa area and in infiltrated cells (arrows) at days 2 and 4 after TNBS administration in iNOS+/+ (B and C) and iNOS−/− mice (F and G). A representative microphotograph at day 7 shows the presence of constitutive ICAM-1 staining in vessels (arrow heads) of the healing mucosa in iNOS+/+ (D) and iNOS−/− mice (H). Original magnification × 400.
Figure 6 .
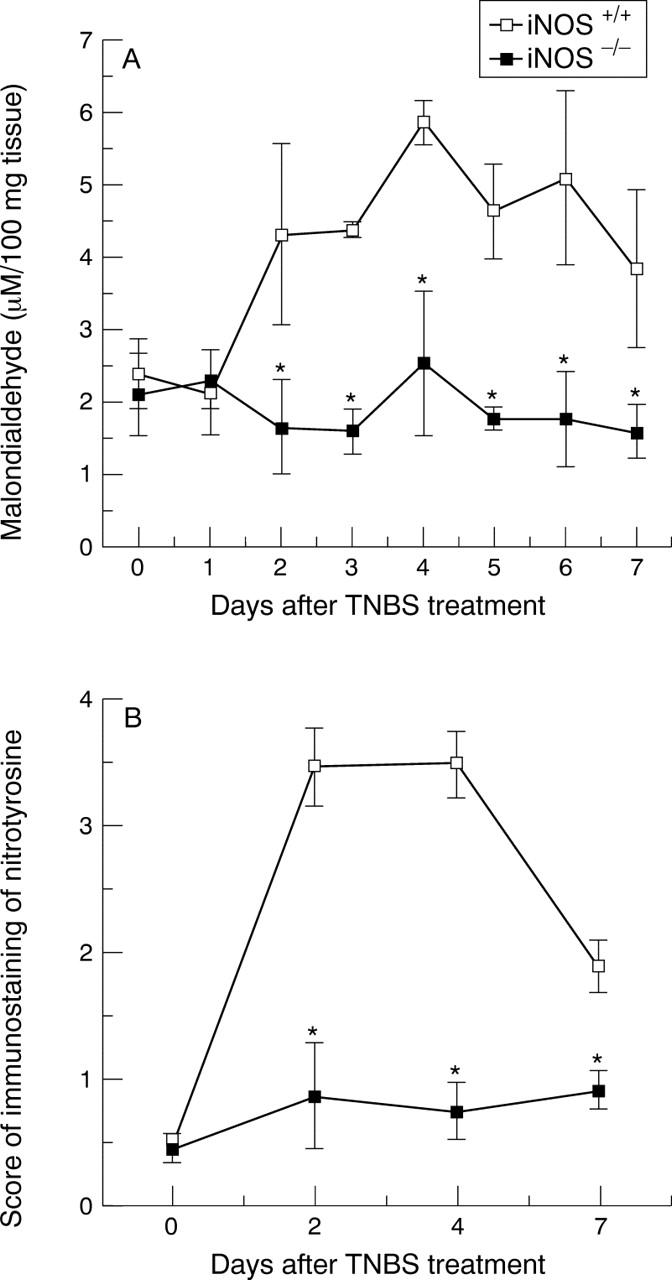
Time course of colonic concentrations of malondialdehyde (A) and score of immunostaining of nitrotyrosine (B) in tissues from TNBS treated iNOS+/+ and iNOS−/− mice. Each data point is the mean (SEM) of eight animals for each group. *p<0.05.
Figure 7 .
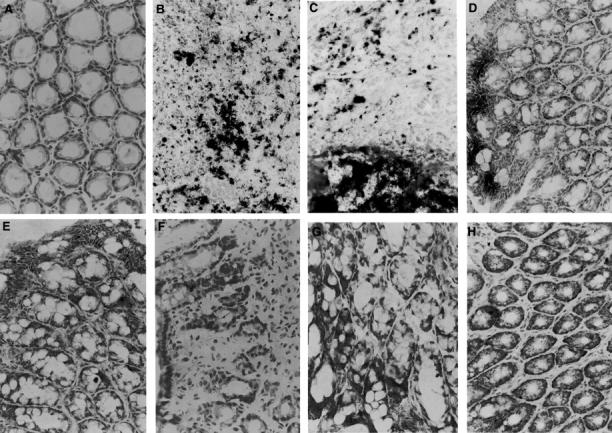
Immunohistochemistry for nitrotyrosine. In colonic sections from non-treated mice (day 0) no staining for nitrotyrosine was found in iNOS+/+ (A) and iNOS−/− mice (E). At days 2 (B) and 4 (C) after TNBS administration a diffuse dark staining was localised in infiltrated inflammatory cells and in the necrotic epithelium of an iNOS+/+mouse. At day 7 (D) nitrotyrosine staining was still present in the apical epithelium in the area of healing mucosa of an iNOS+/+mouse. Nitrotyrosine staining was absent at days 2 (F), 4 (G), and 7 (H) after TNBS administration in the mucosa of an iNOS−/− mouse. Original magnification × 400.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiko S., Grisham M. B. Spontaneous intestinal inflammation and nitric oxide metabolism in HLA-B27 transgenic rats. Gastroenterology. 1995 Jul;109(1):142–150. doi: 10.1016/0016-5085(95)90279-1. [DOI] [PubMed] [Google Scholar]
- Alican I., Kubes P. A critical role for nitric oxide in intestinal barrier function and dysfunction. Am J Physiol. 1996 Feb;270(2 Pt 1):G225–G237. doi: 10.1152/ajpgi.1996.270.2.G225. [DOI] [PubMed] [Google Scholar]
- Beckman J. S. Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Toxicol. 1996 Jul-Aug;9(5):836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- Binion D. G., West G. A., Ina K., Ziats N. P., Emancipator S. N., Fiocchi C. Enhanced leukocyte binding by intestinal microvascular endothelial cells in inflammatory bowel disease. Gastroenterology. 1997 Jun;112(6):1895–1907. doi: 10.1053/gast.1997.v112.pm9178682. [DOI] [PubMed] [Google Scholar]
- Boughton-Smith N. K., Evans S. M., Hawkey C. J., Cole A. T., Balsitis M., Whittle B. J., Moncada S. Nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Lancet. 1993 Aug 7;342(8867):338–340. doi: 10.1016/0140-6736(93)91476-3. [DOI] [PubMed] [Google Scholar]
- Buell M. G., Berin M. C. Neutrophil-independence of the initiation of colonic injury. Comparison of results from three models of experimental colitis in the rat. Dig Dis Sci. 1994 Dec;39(12):2575–2588. doi: 10.1007/BF02087693. [DOI] [PubMed] [Google Scholar]
- Drapier J. C. Interplay between NO and [Fe-S] clusters: relevance to biological systems. Methods. 1997 Mar;11(3):319–329. doi: 10.1006/meth.1996.0426. [DOI] [PubMed] [Google Scholar]
- Eiserich J. P., Cross C. E., Jones A. D., Halliwell B., van der Vliet A. Formation of nitrating and chlorinating species by reaction of nitrite with hypochlorous acid. A novel mechanism for nitric oxide-mediated protein modification. J Biol Chem. 1996 Aug 9;271(32):19199–19208. doi: 10.1074/jbc.271.32.19199. [DOI] [PubMed] [Google Scholar]
- Eiserich J. P., Hristova M., Cross C. E., Jones A. D., Freeman B. A., Halliwell B., van der Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998 Jan 22;391(6665):393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- Elsner J., Sach M., Knopf H. P., Norgauer J., Kapp A., Schollmeyer P., Dobos G. J. Synthesis and surface expression of ICAM-1 in polymorphonuclear neutrophilic leukocytes in normal subjects and during inflammatory disease. Immunobiology. 1995 Aug;193(5):456–464. doi: 10.1016/s0171-2985(11)80430-4. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Sartor R. B., Tennyson G. S., Riddell R. H. Experimental models of inflammatory bowel disease. Gastroenterology. 1995 Oct;109(4):1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- Fenyk-Melody J. E., Garrison A. E., Brunnert S. R., Weidner J. R., Shen F., Shelton B. A., Mudgett J. S. Experimental autoimmune encephalomyelitis is exacerbated in mice lacking the NOS2 gene. J Immunol. 1998 Mar 15;160(6):2940–2946. [PubMed] [Google Scholar]
- Gadelha F. R., Thomson L., Fagian M. M., Costa A. D., Radi R., Vercesi A. E. Ca2+-independent permeabilization of the inner mitochondrial membrane by peroxynitrite is mediated by membrane protein thiol cross-linking and lipid peroxidation. Arch Biochem Biophys. 1997 Sep 15;345(2):243–250. doi: 10.1006/abbi.1997.0259. [DOI] [PubMed] [Google Scholar]
- Gilkeson G. S., Mudgett J. S., Seldin M. F., Ruiz P., Alexander A. A., Misukonis M. A., Pisetsky D. S., Weinberg J. B. Clinical and serologic manifestations of autoimmune disease in MRL-lpr/lpr mice lacking nitric oxide synthase type 2. J Exp Med. 1997 Aug 4;186(3):365–373. doi: 10.1084/jem.186.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham M. B. Oxidants and free radicals in inflammatory bowel disease. Lancet. 1994 Sep 24;344(8926):859–861. doi: 10.1016/s0140-6736(94)92831-2. [DOI] [PubMed] [Google Scholar]
- Halliwell B. What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite formation in vivo? FEBS Lett. 1997 Jul 14;411(2-3):157–160. doi: 10.1016/s0014-5793(97)00469-9. [DOI] [PubMed] [Google Scholar]
- Hickey M. J., Sharkey K. A., Sihota E. G., Reinhardt P. H., Macmicking J. D., Nathan C., Kubes P. Inducible nitric oxide synthase-deficient mice have enhanced leukocyte-endothelium interactions in endotoxemia. FASEB J. 1997 Oct;11(12):955–964. doi: 10.1096/fasebj.11.12.9337148. [DOI] [PubMed] [Google Scholar]
- Hogaboam C. M., Jacobson K., Collins S. M., Blennerhassett M. G. The selective beneficial effects of nitric oxide inhibition in experimental colitis. Am J Physiol. 1995 Apr;268(4 Pt 1):G673–G684. doi: 10.1152/ajpgi.1995.268.4.G673. [DOI] [PubMed] [Google Scholar]
- Ikeda I., Kasajima T., Ishiyama S., Shimojo T., Takeo Y., Nishikawa T., Kameoka S., Hiroe M., Mitsunaga A. Distribution of inducible nitric oxide synthase in ulcerative colitis. Am J Gastroenterol. 1997 Aug;92(8):1339–1341. [PubMed] [Google Scholar]
- Kennedy M., Denenberg A. G., Szabó C., Salzman A. L. Poly(ADP-ribose) synthetase activation mediates increased permeability induced by peroxynitrite in Caco-2BBe cells. Gastroenterology. 1998 Mar;114(3):510–518. doi: 10.1016/s0016-5085(98)70534-7. [DOI] [PubMed] [Google Scholar]
- Khan B. V., Harrison D. G., Olbrych M. T., Alexander R. W., Medford R. M. Nitric oxide regulates vascular cell adhesion molecule 1 gene expression and redox-sensitive transcriptional events in human vascular endothelial cells. Proc Natl Acad Sci U S A. 1996 Aug 20;93(17):9114–9119. doi: 10.1073/pnas.93.17.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I. A., Schwartzman J. D., Matsuura T., Kasper L. H. A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc Natl Acad Sci U S A. 1997 Dec 9;94(25):13955–13960. doi: 10.1073/pnas.94.25.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H., Hokari R., Miura S., Shigematsu T., Hirokawa M., Akiba Y., Kurose I., Higuchi H., Fujimori H., Tsuzuki Y. Increased expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in colonic mucosa of patients with active ulcerative colitis. Gut. 1998 Feb;42(2):180–187. doi: 10.1136/gut.42.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss J., Lamarque D., Delchier J. C., Whittle B. J. Time-dependent actions of nitric oxide synthase inhibition on colonic inflammation induced by trinitrobenzene sulphonic acid in rats. Eur J Pharmacol. 1997 Oct 8;336(2-3):219–224. doi: 10.1016/s0014-2999(97)01246-6. [DOI] [PubMed] [Google Scholar]
- Koizumi M., King N., Lobb R., Benjamin C., Podolsky D. K. Expression of vascular adhesion molecules in inflammatory bowel disease. Gastroenterology. 1992 Sep;103(3):840–847. doi: 10.1016/0016-5085(92)90015-q. [DOI] [PubMed] [Google Scholar]
- Kolios G., Rooney N., Murphy C. T., Robertson D. A., Westwick J. Expression of inducible nitric oxide synthase activity in human colon epithelial cells: modulation by T lymphocyte derived cytokines. Gut. 1998 Jul;43(1):56–63. doi: 10.1136/gut.43.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawisz J. E., Sharon P., Stenson W. F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984 Dec;87(6):1344–1350. [PubMed] [Google Scholar]
- Laubach V. E., Shesely E. G., Smithies O., Sherman P. A. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc Natl Acad Sci U S A. 1995 Nov 7;92(23):10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. O., Hellström P. M., Lundberg J. M., Alving K. Greatly increased luminal nitric oxide in ulcerative colitis. Lancet. 1994 Dec 17;344(8938):1673–1674. doi: 10.1016/s0140-6736(94)90460-x. [DOI] [PubMed] [Google Scholar]
- MacMicking J. D., Nathan C., Hom G., Chartrain N., Fletcher D. S., Trumbauer M., Stevens K., Xie Q. W., Sokol K., Hutchinson N. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995 May 19;81(4):641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- MacMicking J. D., North R. J., LaCourse R., Mudgett J. S., Shah S. K., Nathan C. F. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci U S A. 1997 May 13;94(10):5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNaughton W. K., Cirino G., Wallace J. L. Endothelium-derived relaxing factor (nitric oxide) has protective actions in the stomach. Life Sci. 1989;45(20):1869–1876. doi: 10.1016/0024-3205(89)90540-7. [DOI] [PubMed] [Google Scholar]
- McCafferty D. M., Mudgett J. S., Swain M. G., Kubes P. Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology. 1997 Mar;112(3):1022–1027. doi: 10.1053/gast.1997.v112.pm9041266. [DOI] [PubMed] [Google Scholar]
- McKenzie S. J., Baker M. S., Buffinton G. D., Doe W. F. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest. 1996 Jul 1;98(1):136–141. doi: 10.1172/JCI118757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton S. J., Shorthouse M., Hunter J. O. Increased nitric oxide synthesis in ulcerative colitis. Lancet. 1993 Feb 20;341(8843):465–466. doi: 10.1016/0140-6736(93)90211-x. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Sadowska-Krowicka H., Chotinaruemol S., Kakkis J. L., Clark D. A. Amelioration of chronic ileitis by nitric oxide synthase inhibition. J Pharmacol Exp Ther. 1993 Jan;264(1):11–16. [PubMed] [Google Scholar]
- Miller M. J., Thompson J. H., Zhang X. J., Sadowska-Krowicka H., Kakkis J. L., Munshi U. K., Sandoval M., Rossi J. L., Eloby-Childress S., Beckman J. S. Role of inducible nitric oxide synthase expression and peroxynitrite formation in guinea pig ileitis. Gastroenterology. 1995 Nov;109(5):1475–1483. doi: 10.1016/0016-5085(95)90633-9. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Thompson J. H., Zhang X. J., Sadowska-Krowicka H., Kakkis J. L., Munshi U. K., Sandoval M., Rossi J. L., Eloby-Childress S., Beckman J. S. Role of inducible nitric oxide synthase expression and peroxynitrite formation in guinea pig ileitis. Gastroenterology. 1995 Nov;109(5):1475–1483. doi: 10.1016/0016-5085(95)90633-9. [DOI] [PubMed] [Google Scholar]
- Morris G. P., Beck P. L., Herridge M. S., Depew W. T., Szewczuk M. R., Wallace J. L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989 Mar;96(3):795–803. [PubMed] [Google Scholar]
- Mourelle M., Vilaseca J., Guarner F., Salas A., Malagelada J. R. Toxic dilatation of colon in a rat model of colitis is linked to an inducible form of nitric oxide synthase. Am J Physiol. 1996 Mar;270(3 Pt 1):G425–G430. doi: 10.1152/ajpgi.1996.270.3.G425. [DOI] [PubMed] [Google Scholar]
- Neurath M. F., Fuss I., Kelsall B. L., Stüber E., Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995 Nov 1;182(5):1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979 Jun;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Ohtani H., Nakamura S., Watanabe Y., Fukushima K., Mizoi T., Kimura M., Hiwatashi N., Nagura H. Light and electron microscopic immunolocalization of endothelial leucocyte adhesion molecule-1 in inflammatory bowel disease. Morphological evidence of active synthesis and secretion into vascular lumen. Virchows Arch A Pathol Anat Histopathol. 1992;420(5):403–409. doi: 10.1007/BF01600511. [DOI] [PubMed] [Google Scholar]
- Oshitani N., Campbell A., Bloom S., Kitano A., Kobayashi K., Jewell D. P. Adhesion molecule expression on vascular endothelium and nitroblue tetrazolium reducing activity in human colonic mucosa. Scand J Gastroenterol. 1995 Sep;30(9):915–920. doi: 10.3109/00365529509101601. [DOI] [PubMed] [Google Scholar]
- Parkos C. A., Colgan S. P., Diamond M. S., Nusrat A., Liang T. W., Springer T. A., Madara J. L. Expression and polarization of intercellular adhesion molecule-1 on human intestinal epithelia: consequences for CD11b/CD18-mediated interactions with neutrophils. Mol Med. 1996 Jul;2(4):489–505. [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer C. J., Qiu B. S. Effects of chronic nitric oxide synthase inhibition on TNB-induced colitis in rats. J Pharm Pharmacol. 1995 Oct;47(10):827–832. doi: 10.1111/j.2042-7158.1995.tb05749.x. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D., Karmeli F., Okon E. Sulfhydryl blocker-induced rat colonic inflammation is ameliorated by inhibition of nitric oxide synthase. Gastroenterology. 1995 Jul;109(1):98–106. doi: 10.1016/0016-5085(95)90273-2. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D., Stamler J. S., Karmeli F., Mullins M. E., Singel D. J., Loscalzo J., Xavier R. J., Podolsky D. K. Peroxynitrite-induced rat colitis--a new model of colonic inflammation. Gastroenterology. 1993 Dec;105(6):1681–1688. doi: 10.1016/0016-5085(93)91063-n. [DOI] [PubMed] [Google Scholar]
- Reif D. W. Ferritin as a source of iron for oxidative damage. Free Radic Biol Med. 1992;12(5):417–427. doi: 10.1016/0891-5849(92)90091-t. [DOI] [PubMed] [Google Scholar]
- Rubbo H., Radi R., Trujillo M., Telleri R., Kalyanaraman B., Barnes S., Kirk M., Freeman B. A. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem. 1994 Oct 21;269(42):26066–26075. [PubMed] [Google Scholar]
- Salzman A. L. Nitric oxide in the gut. New Horiz. 1995 Feb;3(1):33–45. [PubMed] [Google Scholar]
- Sekizuka E., Grisham M. B., Li M. A., Deitch E. A., Granger D. N. Inflammation-induced intestinal hyperemia in the rat: role of neutrophils. Gastroenterology. 1988 Dec;95(6):1528–1534. doi: 10.1016/s0016-5085(88)80073-8. [DOI] [PubMed] [Google Scholar]
- Shiratora Y., Aoki S., Takada H., Kiriyama H., Ohto K., Hai K., Teraoka H., Matano S., Matsumoto K., Kamii K. Oxygen-derived free radical generating capacity of polymorphonuclear cells in patients with ulcerative colitis. Digestion. 1989;44(3):163–171. doi: 10.1159/000199906. [DOI] [PubMed] [Google Scholar]
- Simmonds N. J., Allen R. E., Stevens T. R., Van Someren R. N., Blake D. R., Rampton D. S. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology. 1992 Jul;103(1):186–196. doi: 10.1016/0016-5085(92)91112-h. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Kawka D. W., Scott S., Weidner J. R., Mumford R. A., Riehl T. E., Stenson W. F. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology. 1996 Oct;111(4):871–885. doi: 10.1016/s0016-5085(96)70055-0. [DOI] [PubMed] [Google Scholar]
- Stark M. E., Szurszewski J. H. Role of nitric oxide in gastrointestinal and hepatic function and disease. Gastroenterology. 1992 Dec;103(6):1928–1949. doi: 10.1016/0016-5085(92)91454-c. [DOI] [PubMed] [Google Scholar]
- Traylor L. A., Mayeux P. R. Nitric oxide generation mediates lipid A-induced oxidant injury in renal proximal tubules. Arch Biochem Biophys. 1997 Feb 15;338(2):129–135. doi: 10.1006/abbi.1996.9840. [DOI] [PubMed] [Google Scholar]
- Wei X. Q., Charles I. G., Smith A., Ure J., Feng G. J., Huang F. P., Xu D., Muller W., Moncada S., Liew F. Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995 Jun 1;375(6530):408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- Yamada T., Sartor R. B., Marshall S., Specian R. D., Grisham M. B. Mucosal injury and inflammation in a model of chronic granulomatous colitis in rats. Gastroenterology. 1993 Mar;104(3):759–771. doi: 10.1016/0016-5085(93)91011-6. [DOI] [PubMed] [Google Scholar]
- Yamada T., Zimmerman B. J., Specian R. D., Grisham M. B. Role of neutrophils in acetic acid-induced colitis in rats. Inflammation. 1991 Oct;15(5):399–411. doi: 10.1007/BF00917356. [DOI] [PubMed] [Google Scholar]
- Yang H., Vora D. K., Targan S. R., Toyoda H., Beaudet A. L., Rotter J. I. Intercellular adhesion molecule 1 gene associations with immunologic subsets of inflammatory bowel disease. Gastroenterology. 1995 Aug;109(2):440–448. doi: 10.1016/0016-5085(95)90331-3. [DOI] [PubMed] [Google Scholar]
- Yasmin W., Strynadka K. D., Schulz R. Generation of peroxynitrite contributes to ischemia-reperfusion injury in isolated rat hearts. Cardiovasc Res. 1997 Feb;33(2):422–432. doi: 10.1016/s0008-6363(96)00254-4. [DOI] [PubMed] [Google Scholar]
- Zingarelli B., Cuzzocrea S., Szabó C., Salzman A. L. Mercaptoethylguanidine, a combined inhibitor of nitric oxide synthase and peroxynitrite scavenger, reduces trinitrobenzene sulfonic acid-induced colonic damage in rats. J Pharmacol Exp Ther. 1998 Dec;287(3):1048–1055. [PubMed] [Google Scholar]
- Zingarelli B., O'Connor M., Wong H., Salzman A. L., Szabó C. Peroxynitrite-mediated DNA strand breakage activates poly-adenosine diphosphate ribosyl synthetase and causes cellular energy depletion in macrophages stimulated with bacterial lipopolysaccharide. J Immunol. 1996 Jan 1;156(1):350–358. [PubMed] [Google Scholar]
- Zingarelli B., Squadrito F., Graziani P., Camerini R., Caputi A. P. Effects of zileuton, a new 5-lipoxygenase inhibitor, in experimentally induced colitis in rats. Agents Actions. 1993 Jul;39(3-4):150–156. doi: 10.1007/BF01998968. [DOI] [PubMed] [Google Scholar]