Abstract
BACKGROUND—Nitric oxide production by the inducible isoform of nitric oxide synthase (iNOS) is thought to play a role in the pathogenesis of inflammatory bowel disease along with other proinflammatory mediators. AIMS—To examine the effects of cAMP, an intracellular mediator of several proinflammatory mediators, on iNOS expression in the human intestinal epithelial cell line, DLD-1. METHODS—iNOS activity was assessed by measuring the NO stable oxidative product NO2 . iNOS protein expression and iNOS mRNA levels were determined by western blotting and northern blotting, respectively. RESULTS—iNOS activity, protein, and mRNA were induced by a combination of interleukin 1β (0.5-5 ng/ml), interferon γ (20-200 u/ml), and tumour necrosis factor α (10-100 ng/ml). The cytokine induced NOS activity was potentiated by N6,2'-O-dibutyryladenosine 3':5'-cyclic monophosphate and 8-bromoadenosine 3':5'-cyclic monophosphate (0.1-1 mM), and the adenylate cyclase activator, forskolin (1-100 µM). This activity was inhibited by the selective iNOS inhibitor, 1400W (0.1-100 µM). These agents increased iNOS protein. The cAMP analogues potentiated iNOS at the transcriptional level as shown by effects of actinomycin D (5 µg/ml) and northern blot analyses; the nuclear factor (NF) κB inhibitor, pyrrolidine dithiocarbamate (10-200 µM), significantly reduced this potentiation. The cAMP potentiated iNOS activity was inhibited by the tyrosine kinase inhibitor, A25 (10-200 µM) and the Janus activated kinase 2 inhibitor, B42 (10-200 µM). CONCLUSIONS—Increased intracellular cAMP is a potent stimulus of iNOS expression in combination with cytokines in DLD-1 cells, acting at the transcriptional level and involving NF-κB and the JAK-STAT pathways. Thus, proinflammatory mediators that increase cAMP levels may augment iNOS expression and NO production. Keywords: inducible nitric oxide synthase colonic epithelial cells; nuclear factor κB; cytokines; cyclic AMP; JAK-2
Full Text
The Full Text of this article is available as a PDF (133.6 KB).
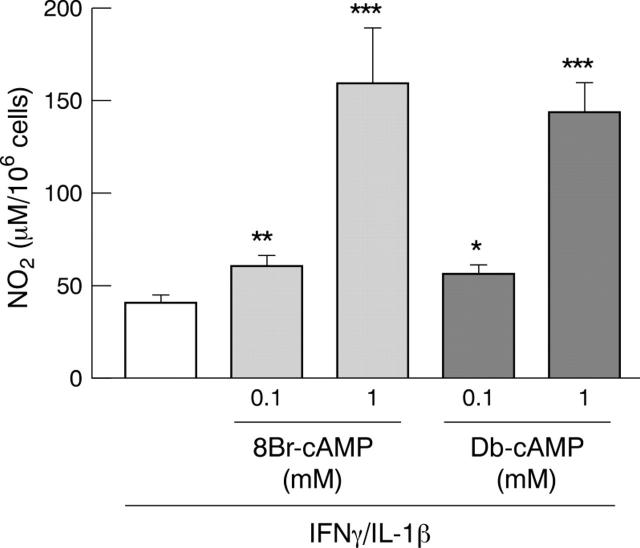
Figure 1 .
Potentiation of IFN-γ/IL-1β induced iNOS activity by 8Br-cAMP and Db-cAMP in DLD-1 cells. Cells were incubated in serum free medium with a combination of IFN-γ and IL-1β alone or with 8Br-cAMP or Db-cAMP. Results are expressed as means (SEM) from at least three different experiments, each done in triplicate. *p<0.05, **p<0.01, ***p<0.001 compared with value in the cells treated by IFN-γ/IL-1β.
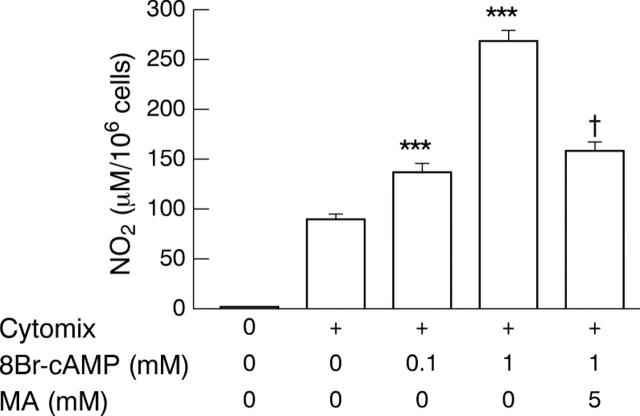
Figure 2 .
Potentiation of cytomix induced iNOS activity by 8Br-cAMP was reversed by the cAMP antagonist, 2'-O-methyl adenosine (MA), in DLD-1 cells. Cells were treated with vehicle alone or cytomix and coincubated with 8Br-cAMP alone or MA. Results are expressed as means (SEM) from at least three different experiments, each done in triplicate. ***p<0.001 compared with value in the cells treated by cytomix alone; †p<0.001 compared with value obtained in the cells treated by cytomix and 8Br-cAMP.
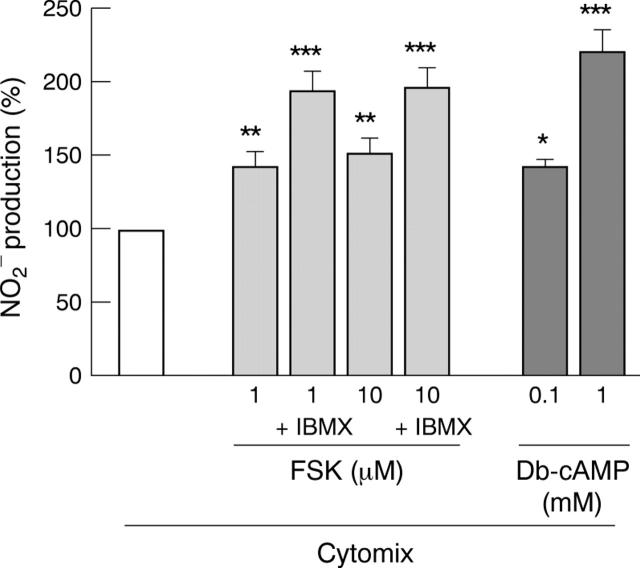
Figure 3 .
Potentiation of cytomix induced iNOS activity by forskolin (FSK) and Db-cAMP in DLD-1 cells. Cells were incubated in serum free medium with cytomix alone (white bars) or FSK, with or without 3-isobutyl-1-methylxanthine (IBMX) or Db-cAMP. Results are expressed as percentage of induction with cytomix alone and represent means (SEM) from at least three different experiments, each done in triplicate. *p<0.05, **p<0.01, ***p<0.001 compared with value obtained in cytomix treated cells.
Figure 4 .
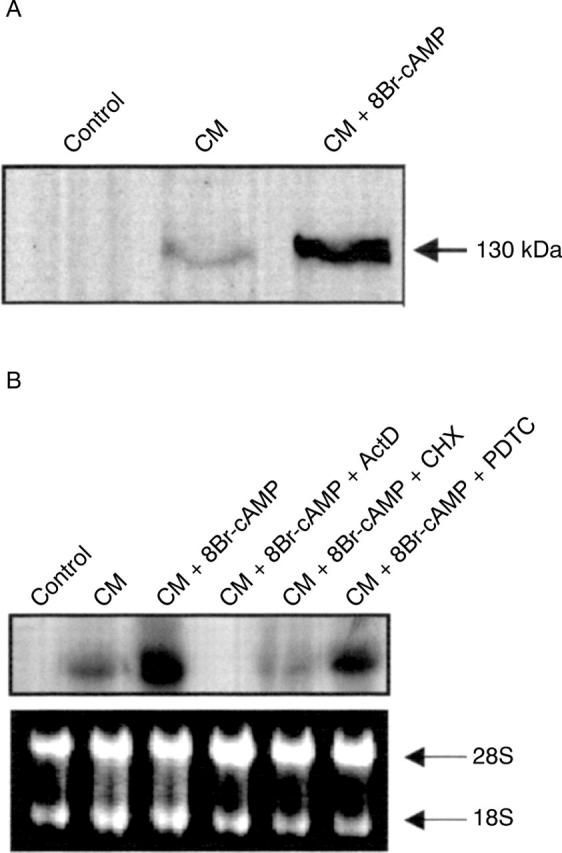
(A) iNOS protein expression in DLD-1 cells. Cells were treated for 12 hours with vehicle alone (control), cytomix (CM), and CM + 8Br-cAMP. This is a representative of three different experiments. (B) Transcriptional control of cAMP potentiation of iNOS induction in DLD-1 cells. Cells were treated for six hours with vehicle alone, CM, CM + 8Br-cAMP, CM + 8Br-cAMP + actinomycin D (ActD), CM + 8Br-cAMP + cycloheximide (CHX), and CM + 8Br-cAMP + pyrrolidine dithiocarbamate (PDTC) (200 µM, two hours pretreatment). (Upper) Northern blot; (lower) ethidium bromide staining of 28S and 18S RNA bands indicating equal loading of the lanes. This is representative of three experiments.
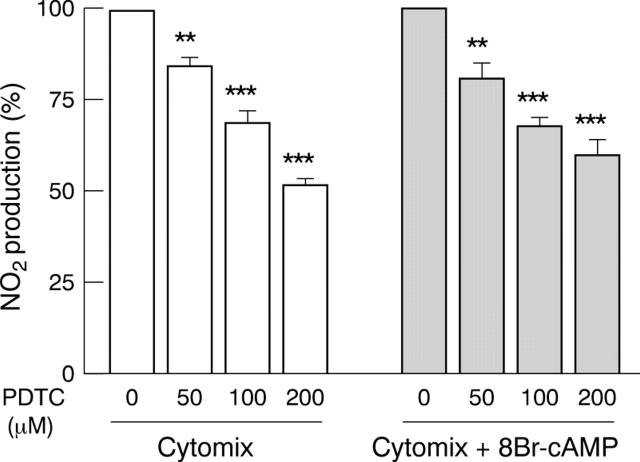
Figure 5 .
Dose dependent inhibition of cytomix induced and cAMP potentiated NO2 production by an NF-κB inhibitor in DLD-1 cells. Cells were treated in serum free medium for 24 hours with cytomix, alone or with 8Br-cAMP, and received two hours' pretreatment with increasing concentrations of pyrrolidine dithiocarbamate (PDTC). Results are expressed as percentage of maximal induction (without PDTC) and represent means (SEM) from at least three different experiments, each done in triplicate. **p<0.01, ***p<0.001 compared with maximal induction.
Figure 6 .
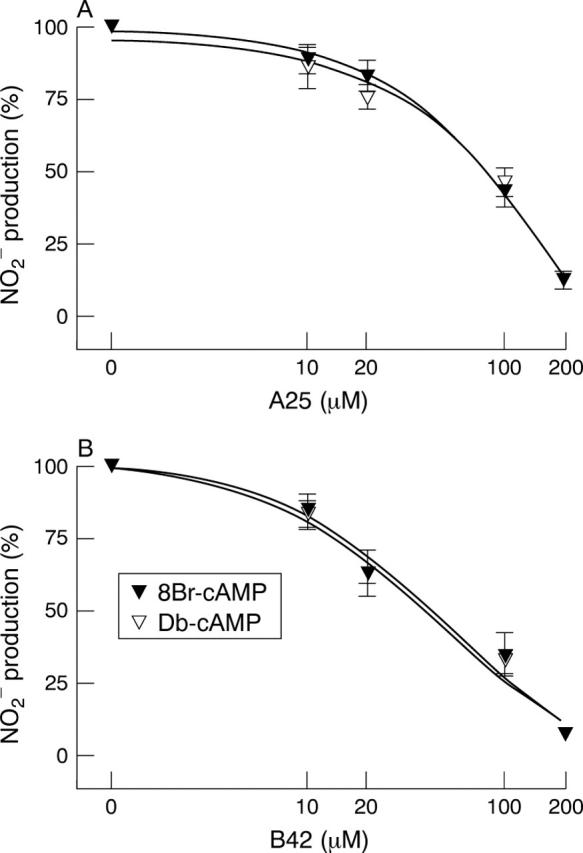
Inhibition of cAMP potentiated NO2 production by the tyrosine kinase inhibitor, tyrphostin A25 (A) and the JAK-2 inhibitor, tyrphostin B42 (B) in DLD-1 cells. Cells were treated in serum free medium for 24 hours with cytomix and 8Br-cAMP or Db-cAMP. Results are expressed as percentage of maximal induction (without tyrphostin) and represent means (SEM) from at least three different experiments, each done in triplicate.
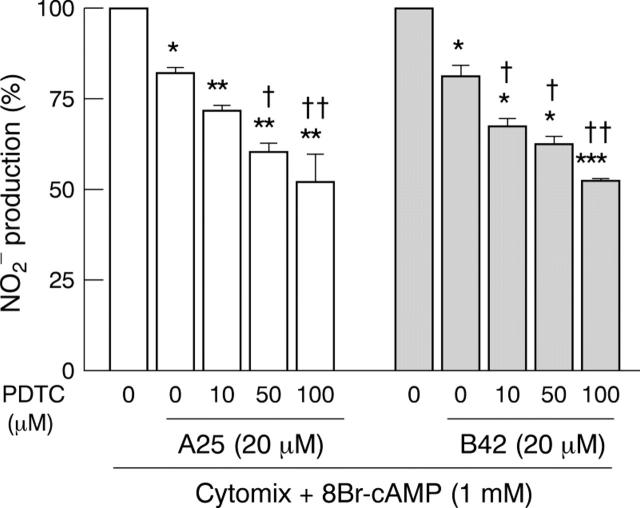
Figure 7 .
Effect of coincubation with the NF-κB inhibitor, pyrrolidine dithiocarbamate (PDTC) and tyrphostins A25 or B42 on cAMP potentiated NO2 production in DLD-1 cells. Cells were treated in serum free medium for 24 hours with cytomix and 8Br-cAMP with or without A25 or B42, and were then treated with increasing concentrations of PDTC. Results are expressed as percentage of maximal induction (without tyrphostin and PDTC) and represent means (SEM) from at least three different experiments, each done in triplicate. *p<0.05, **p<0.01, ***p<0.001, compared with maximal induction; †p<0.05, ††p<0.01, compared with value obtained in cells treated with A25 or B42.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aymerich M. S., Bengoechea-Alonso M. T., López-Zabalza M. J., Santiago E., López-Moratalla N. Inducible nitric oxide synthase (iNOS) expression in human monocytes triggered by beta-endorphin through an increase in cAMP. Biochem Biophys Res Commun. 1998 Apr 28;245(3):717–721. doi: 10.1006/bbrc.1998.8127. [DOI] [PubMed] [Google Scholar]
- Benbernou N., Esnault S., Shin H. C., Fekkar H., Guenounou M. Differential regulation of IFN-gamma, IL-10 and inducible nitric oxide synthase in human T cells by cyclic AMP-dependent signal transduction pathway. Immunology. 1997 Jul;91(3):361–368. doi: 10.1046/j.1365-2567.1997.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode H., Schmitz H., Fromm M., Scholz P., Riecken E. O., Schulzke J. D. IL-1beta and TNF-alpha, but not IFN-alpha, IFN-gamma, IL-6 or IL-8, are secretory mediators in human distal colon. Cytokine. 1998 Jun;10(6):457–465. doi: 10.1006/cyto.1997.0307. [DOI] [PubMed] [Google Scholar]
- Boese M., Busse R., Mülsch A., Schini-Kerth V. Effect of cyclic GMP-dependent vasodilators on the expression of inducible nitric oxide synthase in vascular smooth muscle cells: role of cyclic AMP. Br J Pharmacol. 1996 Oct;119(4):707–715. doi: 10.1111/j.1476-5381.1996.tb15730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughton-Smith N. K., Evans S. M., Hawkey C. J., Cole A. T., Balsitis M., Whittle B. J., Moncada S. Nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Lancet. 1993 Aug 7;342(8867):338–340. doi: 10.1016/0140-6736(93)91476-3. [DOI] [PubMed] [Google Scholar]
- Coffey R. G., Yamamoto Y., Snella E., Pross S. Tetrahydrocannabinol inhibition of macrophage nitric oxide production. Biochem Pharmacol. 1996 Sep 13;52(5):743–751. doi: 10.1016/0006-2952(96)00356-5. [DOI] [PubMed] [Google Scholar]
- Cook H. T., Bune A. J., Jansen A. S., Taylor G. M., Loi R. K., Cattell V. Cellular localization of inducible nitric oxide synthase in experimental endotoxic shock in the rat. Clin Sci (Lond) 1994 Aug;87(2):179–186. doi: 10.1042/cs0870179. [DOI] [PubMed] [Google Scholar]
- Eberhardt W., Plüss C., Hummel R., Pfeilschifter J. Molecular mechanisms of inducible nitric oxide synthase gene expression by IL-1beta and cAMP in rat mesangial cells. J Immunol. 1998 May 15;160(10):4961–4969. [PubMed] [Google Scholar]
- Everest P. H., Cole A. T., Hawkey C. J., Knutton S., Goossens H., Butzler J. P., Ketley J. M., Williams P. H. Roles of leukotriene B4, prostaglandin E2, and cyclic AMP in Campylobacter jejuni-induced intestinal fluid secretion. Infect Immun. 1993 Nov;61(11):4885–4887. doi: 10.1128/iai.61.11.4885-4887.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein D. L., Galea E., Reis D. J. Norepinephrine suppresses inducible nitric oxide synthase activity in rat astroglial cultures. J Neurochem. 1993 May;60(5):1945–1948. doi: 10.1111/j.1471-4159.1993.tb13425.x. [DOI] [PubMed] [Google Scholar]
- Gilbert R. S., Herschman H. R. "Macrophage" nitric oxide synthase is a glucocorticoid-inhibitable primary response gene in 3T3 cells. J Cell Physiol. 1993 Oct;157(1):128–132. doi: 10.1002/jcp.1041570117. [DOI] [PubMed] [Google Scholar]
- Godkin A. J., De Belder A. J., Villa L., Wong A., Beesley J. E., Kane S. P., Martin J. F. Expression of nitric oxide synthase in ulcerative colitis. Eur J Clin Invest. 1996 Oct;26(10):867–872. doi: 10.1111/j.1365-2362.1996.tb02131.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez G. A., Montminy M. R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989 Nov 17;59(4):675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Hendel J., Nielsen O. H. Expression of cyclooxygenase-2 mRNA in active inflammatory bowel disease. Am J Gastroenterol. 1997 Jul;92(7):1170–1173. [PubMed] [Google Scholar]
- Ikeda I., Kasajima T., Ishiyama S., Shimojo T., Takeo Y., Nishikawa T., Kameoka S., Hiroe M., Mitsunaga A. Distribution of inducible nitric oxide synthase in ulcerative colitis. Am J Gastroenterol. 1997 Aug;92(8):1339–1341. [PubMed] [Google Scholar]
- Jeon Y. J., Yang K. H., Pulaski J. T., Kaminski N. E. Attenuation of inducible nitric oxide synthase gene expression by delta 9-tetrahydrocannabinol is mediated through the inhibition of nuclear factor- kappa B/Rel activation. Mol Pharmacol. 1996 Aug;50(2):334–341. [PubMed] [Google Scholar]
- Kimura H., Miura S., Shigematsu T., Ohkubo N., Tsuzuki Y., Kurose I., Higuchi H., Akiba Y., Hokari R., Hirokawa M. Increased nitric oxide production and inducible nitric oxide synthase activity in colonic mucosa of patients with active ulcerative colitis and Crohn's disease. Dig Dis Sci. 1997 May;42(5):1047–1054. doi: 10.1023/a:1018849405922. [DOI] [PubMed] [Google Scholar]
- Kinugawa K., Shimizu T., Yao A., Kohmoto O., Serizawa T., Takahashi T. Transcriptional regulation of inducible nitric oxide synthase in cultured neonatal rat cardiac myocytes. Circ Res. 1997 Dec;81(6):911–921. doi: 10.1161/01.res.81.6.911. [DOI] [PubMed] [Google Scholar]
- Kleinert H., Euchenhofer C., Fritz G., Ihrig-Biedert I., Förstermann U. Involvement of protein kinases in the induction of NO synthase II in human DLD-1 cells. Br J Pharmacol. 1998 Apr;123(8):1716–1722. doi: 10.1038/sj.bjp.0701782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinert H., Wallerath T., Fritz G., Ihrig-Biedert I., Rodriguez-Pascual F., Geller D. A., Förstermann U. Cytokine induction of NO synthase II in human DLD-1 cells: roles of the JAK-STAT, AP-1 and NF-kappaB-signaling pathways. Br J Pharmacol. 1998 Sep;125(1):193–201. doi: 10.1038/sj.bjp.0702039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. G., Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994 Mar 1;298(Pt 2):249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide M., Kawahara Y., Nakayama I., Tsuda T., Yokoyama M. Cyclic AMP-elevating agents induce an inducible type of nitric oxide synthase in cultured vascular smooth muscle cells. Synergism with the induction elicited by inflammatory cytokines. J Biol Chem. 1993 Nov 25;268(33):24959–24966. [PubMed] [Google Scholar]
- Kolios G., Rooney N., Murphy C. T., Robertson D. A., Westwick J. Expression of inducible nitric oxide synthase activity in human colon epithelial cells: modulation by T lymphocyte derived cytokines. Gut. 1998 Jul;43(1):56–63. doi: 10.1136/gut.43.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz D., Mühl H., Walker G., Pfeilschifter J. Two distinct signaling pathways trigger the expression of inducible nitric oxide synthase in rat renal mesangial cells. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5387–5391. doi: 10.1073/pnas.91.12.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritsen K., Laursen L. S., Bukhave K., Rask-Madsen J. Effects of topical 5-aminosalicylic acid and prednisolone on prostaglandin E2 and leukotriene B4 levels determined by equilibrium in vivo dialysis of rectum in relapsing ulcerative colitis. Gastroenterology. 1986 Oct;91(4):837–844. doi: 10.1016/0016-5085(86)90684-0. [DOI] [PubMed] [Google Scholar]
- Linn S. C., Morelli P. J., Edry I., Cottongim S. E., Szabó C., Salzman A. L. Transcriptional regulation of human inducible nitric oxide synthase gene in an intestinal epithelial cell line. Am J Physiol. 1997 Jun;272(6 Pt 1):G1499–G1508. doi: 10.1152/ajpgi.1997.272.6.G1499. [DOI] [PubMed] [Google Scholar]
- Masquilier D., Sassone-Corsi P. Transcriptional cross-talk: nuclear factors CREM and CREB bind to AP-1 sites and inhibit activation by Jun. J Biol Chem. 1992 Nov 5;267(31):22460–22466. [PubMed] [Google Scholar]
- Mauël J., Ransijn A., Corradin S. B., Buchmüller-Rouiller Y. Effect of PGE2 and of agents that raise cAMP levels on macrophage activation induced by IFN-gamma and TNF-alpha. J Leukoc Biol. 1995 Aug;58(2):217–224. doi: 10.1002/jlb.58.2.217. [DOI] [PubMed] [Google Scholar]
- McLaughlan J. M., Seth R., Vautier G., Robins R. A., Scott B. B., Hawkey C. J., Jenkins D. Interleukin-8 and inducible nitric oxide synthase mRNA levels in inflammatory bowel disease at first presentation. J Pathol. 1997 Jan;181(1):87–92. doi: 10.1002/(SICI)1096-9896(199701)181:1<87::AID-PATH736>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Messmer U. K., Brüne B. Modulation of inducible nitric oxide synthase in RINm5F cells. Cell Signal. 1994 Jan;6(1):17–24. doi: 10.1016/0898-6568(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Meydan N., Grunberger T., Dadi H., Shahar M., Arpaia E., Lapidot Z., Leeder J. S., Freedman M., Cohen A., Gazit A. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 1996 Feb 15;379(6566):645–648. doi: 10.1038/379645a0. [DOI] [PubMed] [Google Scholar]
- Minghetti L., Nicolini A., Polazzi E., Créminon C., Maclouf J., Levi G. Prostaglandin E2 downregulates inducible nitric oxide synthase expression in microglia by increasing cAMP levels. Adv Exp Med Biol. 1997;433:181–184. doi: 10.1007/978-1-4899-1810-9_37. [DOI] [PubMed] [Google Scholar]
- Moore A. R., Willoughby D. A. The role of cAMP regulation in controlling inflammation. Clin Exp Immunol. 1995 Sep;101(3):387–389. doi: 10.1111/j.1365-2249.1995.tb03123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroi M., Suzuki T. Role of protein kinase A in LPS-induced activation of NF-kappa B proteins of a mouse macrophage-like cell line, J774. Cell Signal. 1993 May;5(3):289–298. doi: 10.1016/0898-6568(93)90019-i. [DOI] [PubMed] [Google Scholar]
- Mustafa S. B., Olson M. S. Expression of nitric-oxide synthase in rat Kupffer cells is regulated by cAMP. J Biol Chem. 1998 Feb 27;273(9):5073–5080. doi: 10.1074/jbc.273.9.5073. [DOI] [PubMed] [Google Scholar]
- Nunokawa Y., Oikawa S., Tanaka S. Human inducible nitric oxide synthase gene is transcriptionally regulated by nuclear factor-kappaB dependent mechanism. Biochem Biophys Res Commun. 1996 Jun 14;223(2):347–352. doi: 10.1006/bbrc.1996.0897. [DOI] [PubMed] [Google Scholar]
- Nüsing R. M., Klein T., Pfeilschifter J., Ullrich V. Effect of cyclic AMP and prostaglandin E2 on the induction of nitric oxide- and prostanoid-forming pathways in cultured rat mesangial cells. Biochem J. 1996 Jan 15;313(Pt 2):617–623. doi: 10.1042/bj3130617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudkerk Pool M., Bouma G., Visser J. J., Kolkman J. J., Tran D. D., Meuwissen S. G., Peña A. S. Serum nitrate levels in ulcerative colitis and Crohn's disease. Scand J Gastroenterol. 1995 Aug;30(8):784–788. doi: 10.3109/00365529509096328. [DOI] [PubMed] [Google Scholar]
- Pang L., Hoult J. R. Repression of inducible nitric oxide synthase and cyclooxygenase-2 by prostaglandin E2 and other cyclic AMP stimulants in J774 macrophages. Biochem Pharmacol. 1997 Feb 21;53(4):493–500. doi: 10.1016/s0006-2952(96)00737-x. [DOI] [PubMed] [Google Scholar]
- Peterson J. W., Lu Y., Duncan S., Cantu J., Chopra A. K. Interactions of intestinal mediators in the mode of action of cholera toxin. J Med Microbiol. 1994 Jul;41(1):3–9. doi: 10.1099/00222615-41-1-3. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D., Karmeli F., Selinger Z. Increased colonic adenylate cyclase activity in active ulcerative colitis. Gastroenterology. 1983 Jul;85(1):12–16. [PubMed] [Google Scholar]
- Rachmilewitz D., Stamler J. S., Bachwich D., Karmeli F., Ackerman Z., Podolsky D. K. Enhanced colonic nitric oxide generation and nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Gut. 1995 May;36(5):718–723. doi: 10.1136/gut.36.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman A., Denenberg A. G., Ueta I., O'Connor M., Linn S. C., Szabó C. Induction and activity of nitric oxide synthase in cultured human intestinal epithelial monolayers. Am J Physiol. 1996 Apr;270(4 Pt 1):G565–G573. doi: 10.1152/ajpgi.1996.270.4.G565. [DOI] [PubMed] [Google Scholar]
- Serkkola E., Hurme M. Activation of NF-kappa B by cAMP in human myeloid cells. FEBS Lett. 1993 Nov 22;334(3):327–330. doi: 10.1016/0014-5793(93)80704-x. [DOI] [PubMed] [Google Scholar]
- Sherman P. A., Laubach V. E., Reep B. R., Wood E. R. Purification and cDNA sequence of an inducible nitric oxide synthase from a human tumor cell line. Biochemistry. 1993 Nov 2;32(43):11600–11605. doi: 10.1021/bi00094a017. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Kawka D. W., Schloemann S., Tessner T., Riehl T., Stenson W. F. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998 Aug;115(2):297–306. doi: 10.1016/s0016-5085(98)70196-9. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Kawka D. W., Scott S., Weidner J. R., Mumford R. A., Riehl T. E., Stenson W. F. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology. 1996 Oct;111(4):871–885. doi: 10.1016/s0016-5085(96)70055-0. [DOI] [PubMed] [Google Scholar]
- Smith F. S., Ceppi E. D., Titheradge M. A. Inhibition of cytokine-induced inducible nitric oxide synthase expression by glucagon and cAMP in cultured hepatocytes. Biochem J. 1997 Aug 15;326(Pt 1):187–192. doi: 10.1042/bj3260187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepperman B. L., Brown J. F., Whittle B. J. Nitric oxide synthase induction and intestinal epithelial cell viability in rats. Am J Physiol. 1993 Aug;265(2 Pt 1):G214–G218. doi: 10.1152/ajpgi.1993.265.2.G214. [DOI] [PubMed] [Google Scholar]
- Weber-Nordt R. M., Mertelsmann R., Finke J. The JAK-STAT pathway: signal transduction involved in proliferation, differentiation and transformation. Leuk Lymphoma. 1998 Feb;28(5-6):459–467. doi: 10.3109/10428199809058353. [DOI] [PubMed] [Google Scholar]
- Wu K. K. Inducible cyclooxygenase and nitric oxide synthase. Adv Pharmacol. 1995;33:179–207. doi: 10.1016/s1054-3589(08)60669-9. [DOI] [PubMed] [Google Scholar]