Full Text
The Full Text of this article is available as a PDF (175.8 KB).
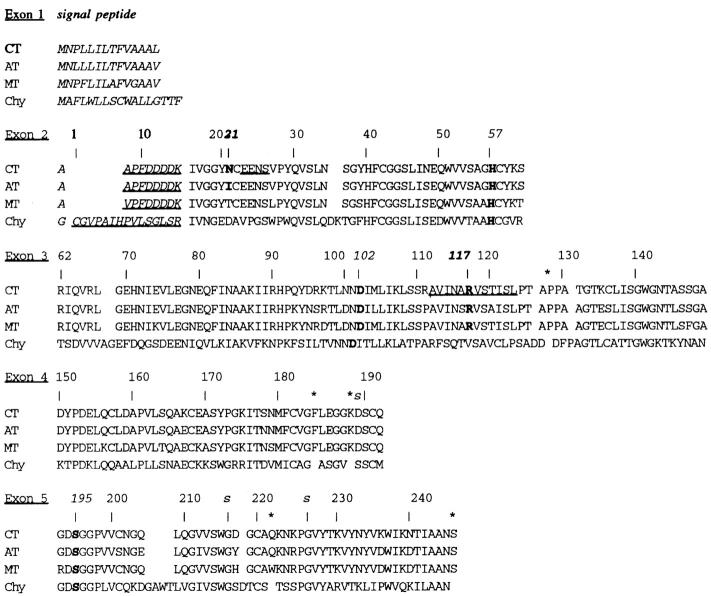
Figure 1 .
Cationic trypsinogen (CT) compared with anionic trypsinogen (AT), mesotrypsinogen (MT) and chymotrypsinogen (Chy). Cationic trypsinogen is divided according to the amino acids coded for in each of the five exons. The numbering system is based on alignment with chymotrypsinogen at serine 195.16 The sequences of cationic and anionic trypsinogen are from17; mesotrypsinogen from18 and19; and chymotrypsinogen from cDNA (introns unknown).20 Signal peptides are in italics, activation peptides are underlined italics, the catalytic triad (H57, D102 and S195), N21, and R117 are bold. The amino acids determining specificity of the enzymes (D189, G216 and G226) are marked with "s". Amino acids in trypsinogen that occur between the chymotrypsinogen numbered residues are marked with an asterisk.
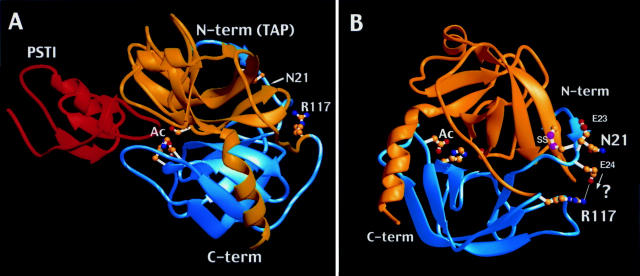
Figure 2 .
Crystallographic structure of trypsin. (A) The two domain structure of bovine trypsinogen-pancreatic secretory trypsin inhibitor (PSTI) complex.35 Note the transition from the blue N-terminal (N-term) domain to the yellow C-terminal (C-term) domain at R117 in the flexible chain segment connecting the domains. The trypsinogen activation peptide (TAP) portion of the molecule resides in the N-term region but is not visualised crystallographically. PSTI (red) is shown interacting with the active site (Ac) of trypsin. The location of N21 is also noted. (B) The two domain structure of human cationic trypsin.21 Structural location of N21, E24, and R117. One possible effect of the N21I substitution would be to bring E24 closer to R117, forming a salt bridge between the oppositely charged side chains (arrow and "?").
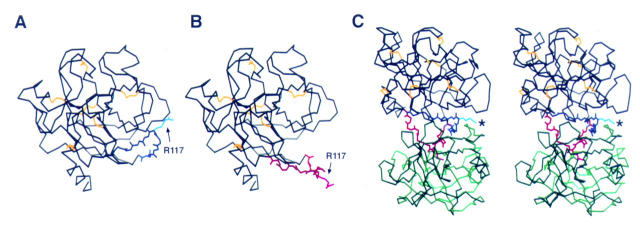
Figure 3 .
Limited proteolysis model of trypsin by trypsin. The backbone of the primary trypsinogen (black) is illustrated with the A112-L123 side chain shown in native conformation (A; blue) and the modelled conformation (B; red) that may be necessary for limited proteolysis of trypsin at R117 (arrow) by another trypsin molecule. (C) Stereo view of the trypsin-trypsin autolysis limited proteolysis model with a second trypsin (green) in the attack position. The two trypsin molecules are oriented with the active site facing upward. The A112-L123 side chain from A and B are both illustrated, with R117 in the native position marked with a asterisk. When the side chain is in the modelled (red) conformation the R117 of the primary (black) trypsin molecule easily fits within the active site of the second trypsin molecule. (To see the molecule in three dimensions, position the figure approximately 30 cm (12 inches) from the face, relaxing and crossing the eyes to allow the images to merge. One should see three images, with the one in the middle appearing in three dimensions.)
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammann R. W., Heitz P. U., Klöppel G. Course of alcoholic chronic pancreatitis: a prospective clinicomorphological long-term study. Gastroenterology. 1996 Jul;111(1):224–231. doi: 10.1053/gast.1996.v111.pm8698203. [DOI] [PubMed] [Google Scholar]
- Apte M. V., Haber P. S., Applegate T. L., Norton I. D., McCaughan G. W., Korsten M. A., Pirola R. C., Wilson J. S. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998 Jul;43(1):128–133. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachem M. G., Schneider E., Gross H., Weidenbach H., Schmid R. M., Menke A., Siech M., Beger H., Grünert A., Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998 Aug;115(2):421–432. doi: 10.1016/s0016-5085(98)70209-4. [DOI] [PubMed] [Google Scholar]
- Bell S. M., Bennett C., Markham A. F., Lench N. J. Evidence for a common mutation in hereditary pancreatitis. Mol Pathol. 1998 Apr;51(2):115–117. doi: 10.1136/mp.51.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone M. Premature trypsin activation in hereditary pancreatitis. Gastroenterology. 1998 Sep;115(3):796–799. doi: 10.1016/s0016-5085(98)70173-8. [DOI] [PubMed] [Google Scholar]
- Bolognesi M., Gatti G., Menagatti E., Guarneri M., Marquart M., Papamokos E., Huber R. Three-dimensional structure of the complex between pancreatic secretory trypsin inhibitor (Kazal type) and trypsinogen at 1.8 A resolution. Structure solution, crystallographic refinement and preliminary structural interpretation. J Mol Biol. 1982 Dec 25;162(4):839–868. doi: 10.1016/0022-2836(82)90550-2. [DOI] [PubMed] [Google Scholar]
- COMFORT M. W., STEINBERG A. G. Pedigree of a family with hereditary chronic relapsing pancreatitis. Gastroenterology. 1952 May;21(1):54–63. [PubMed] [Google Scholar]
- Cavallini G., Tittobello A., Frulloni L., Masci E., Mariana A., Di Francesco V. Gabexate for the prevention of pancreatic damage related to endoscopic retrograde cholangiopancreatography. Gabexate in digestive endoscopy--Italian Group. N Engl J Med. 1996 Sep 26;335(13):919–923. doi: 10.1056/NEJM199609263351302. [DOI] [PubMed] [Google Scholar]
- Colomb E., Figarella C. Comparative studies on the mechanism of activation of the two human trypsinogens. Biochim Biophys Acta. 1979 Dec 7;571(2):343–351. doi: 10.1016/0005-2744(79)90104-9. [DOI] [PubMed] [Google Scholar]
- Colomb E., Guy O., Deprez P., Michel R., Figarella C. The two human trypsinogens: catalytic properties of the corresponding trypsins. Biochim Biophys Acta. 1978 Jul 7;525(1):186–193. doi: 10.1016/0005-2744(78)90213-9. [DOI] [PubMed] [Google Scholar]
- Comfort M. W., Gambrill E. E., Baggenstoss A. H. Chronic relapsing pancreatitis. A study of twenty-nine cases without associated disease of the biliary or gastro-intestinal tract. Gastroenterology. 1968 Apr;54(4 Suppl):760–765. [PubMed] [Google Scholar]
- Dasouki M. J., Cogan J., Summar M. L., Neblitt W., 3rd, Foroud T., Koller D., Phillips J. A., 3rd Heterogeneity in hereditary pancreatitis. Am J Med Genet. 1998 Apr 28;77(1):47–53. [PubMed] [Google Scholar]
- Durie P. R. Pancreatitis and mutations of the cystic fibrosis gene. N Engl J Med. 1998 Sep 3;339(10):687–688. doi: 10.1056/NEJM199809033391008. [DOI] [PubMed] [Google Scholar]
- Figarella C., Amouric M., Guy-Crotte O. Proteolysis of human trypsinogen 1. Pathogenic implication in chronic pancreatitis. Biochem Biophys Res Commun. 1984 Jan 13;118(1):154–161. doi: 10.1016/0006-291x(84)91080-5. [DOI] [PubMed] [Google Scholar]
- Figarella C., Miszczuk-Jamska B., Barrett A. J. Possible lysosomal activation of pancreatic zymogens. Activation of both human trypsinogens by cathepsin B and spontaneous acid. Activation of human trypsinogen 1. Biol Chem Hoppe Seyler. 1988 May;369 (Suppl):293–298. [PubMed] [Google Scholar]
- Freedman S. D. New concepts in understanding the pathophysiology of chronic pancreatitis. Int J Pancreatol. 1998 Aug;24(1):1–8. doi: 10.1007/BF02787524. [DOI] [PubMed] [Google Scholar]
- Frick T. W., Fernández-del Castillo C., Bimmler D., Warshaw A. L. Elevated calcium and activation of trypsinogen in rat pancreatic acini. Gut. 1997 Sep;41(3):339–343. doi: 10.1136/gut.41.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried A. M., Selke A. C. Pseudocyst formation in hereditary pancreatitis. J Pediatr. 1978 Dec;93(6):950–953. doi: 10.1016/s0022-3476(78)81217-7. [DOI] [PubMed] [Google Scholar]
- GREENBAUM L. M., HIRSHKOWITZ A., SHOICHET I. The activation of trypsinogen by cathepsin B. J Biol Chem. 1959 Nov;234:2885–2890. [PubMed] [Google Scholar]
- Gaboriaud C., Serre L., Guy-Crotte O., Forest E., Fontecilla-Camps J. C. Crystal structure of human trypsin 1: unexpected phosphorylation of Tyr151. J Mol Biol. 1996 Jun 28;259(5):995–1010. doi: 10.1006/jmbi.1996.0376. [DOI] [PubMed] [Google Scholar]
- Gorry M. C., Gabbaizedeh D., Furey W., Gates L. K., Jr, Preston R. A., Aston C. E., Zhang Y., Ulrich C., Ehrlich G. D., Whitcomb D. C. Mutations in the cationic trypsinogen gene are associated with recurrent acute and chronic pancreatitis. Gastroenterology. 1997 Oct;113(4):1063–1068. doi: 10.1053/gast.1997.v113.pm9322498. [DOI] [PubMed] [Google Scholar]
- Goto M., Matsuno K., Yamaguchi Y., Ezaki T., Ogawa M. Proliferation kinetics of macrophage subpopulations in a rat experimental pancreatitis model. Arch Histol Cytol. 1993 Mar;56(1):75–82. doi: 10.1679/aohc.56.75. [DOI] [PubMed] [Google Scholar]
- Goto M., Nakano I., Kimura T., Miyahara T., Kinjo M., Nawata H. New chronic pancreatitis model with diabetes induced by cerulein plus stress in rats. Dig Dis Sci. 1995 Nov;40(11):2356–2363. doi: 10.1007/BF02063237. [DOI] [PubMed] [Google Scholar]
- Gress T. M., Micha A. E., Lacher U., Adler G. Diagnose einer "hereditären Pankreatitis" durch Nachweis der Mutation im kationischen Trypsinogen-Gen. Dtsch Med Wochenschr. 1998 Apr 9;123(15):453–456. doi: 10.1055/s-2007-1023986. [DOI] [PubMed] [Google Scholar]
- Guy O., Lombardo D., Bartelt D. C., Amic J., Figarella C. Two human trypsinogens. Purification, molecular properties, and N-terminal sequences. Biochemistry. 1978 May 2;17(9):1669–1675. doi: 10.1021/bi00602a014. [DOI] [PubMed] [Google Scholar]
- Hanck C., Singer M. V. Does acute alcoholic pancreatitis exist without preexisting chronic pancreatitis? Scand J Gastroenterol. 1997 Jul;32(7):625–626. doi: 10.3109/00365529708996508. [DOI] [PubMed] [Google Scholar]
- Hubbard S. J., Eisenmenger F., Thornton J. M. Modeling studies of the change in conformation required for cleavage of limited proteolytic sites. Protein Sci. 1994 May;3(5):757–768. doi: 10.1002/pro.5560030505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T., Hayasaka A., Yamaguchi T., Kondo F., Saisho H. Immunohistochemical study of transforming growth factor-beta 1, matrix metalloproteinase-2,9, tissue inhibitors of metalloproteinase-1,2, and basement membrane components at pancreatic ducts in chronic pancreatitis. Pancreas. 1998 Nov;17(4):412–418. doi: 10.1097/00006676-199811000-00013. [DOI] [PubMed] [Google Scholar]
- Josien R., Douillard P., Guillot C., Müschen M., Anegon I., Chetritt J., Menoret S., Vignes C., Soulillou J. P., Cuturi M. C. A critical role for transforming growth factor-beta in donor transfusion-induced allograft tolerance. J Clin Invest. 1998 Dec 1;102(11):1920–1926. doi: 10.1172/JCI4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattwinkel J., Lapey A., Di Sant'Agnese P. A., Edwards W. A. Hereditary pancreatitis: three new kindreds and a critical review of the literature. Pediatrics. 1973 Jan;51(1):55–69. [PubMed] [Google Scholar]
- Kimland M., Russick C., Marks W. H., Borgström A. Immunoreactive anionic and cationic trypsin in human serum. Clin Chim Acta. 1989 Sep 15;184(1):31–46. doi: 10.1016/0009-8981(89)90254-4. [DOI] [PubMed] [Google Scholar]
- Klöppel G., Maillet B. Pathology of acute and chronic pancreatitis. Pancreas. 1993 Nov;8(6):659–670. doi: 10.1097/00006676-199311000-00001. [DOI] [PubMed] [Google Scholar]
- Klöppel G., Maillet B. Pseudocysts in chronic pancreatitis: a morphological analysis of 57 resection specimens and 9 autopsy pancreata. Pancreas. 1991 May;6(3):266–274. [PubMed] [Google Scholar]
- Le Bodic L., Bignon J. D., Raguénès O., Mercier B., Georgelin T., Schnee M., Soulard F., Gagne K., Bonneville F., Muller J. Y. The hereditary pancreatitis gene maps to long arm of chromosome 7. Hum Mol Genet. 1996 Apr;5(4):549–554. doi: 10.1093/hmg/5.4.549. [DOI] [PubMed] [Google Scholar]
- Le Bodic L., Schnee M., Georgelin T., Soulard F., Ferec C., Bignon J. D., Sagniez M. An exceptional genealogy for hereditary chronic pancreatitis. Dig Dis Sci. 1996 Jul;41(7):1504–1510. doi: 10.1007/BF02088580. [DOI] [PubMed] [Google Scholar]
- Leach S. D., Modlin I. M., Scheele G. A., Gorelick F. S. Intracellular activation of digestive zymogens in rat pancreatic acini. Stimulation by high doses of cholecystokinin. J Clin Invest. 1991 Jan;87(1):362–366. doi: 10.1172/JCI114995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy R., Christiansen P. A. Hereditary pancreatitis in a kinship associated with portal vein thrombosis. Am J Med. 1972 Feb;52(2):228–241. doi: 10.1016/0002-9343(72)90072-1. [DOI] [PubMed] [Google Scholar]
- Mithöfer K., Fernández-del Castillo C., Frick T. W., Lewandrowski K. B., Rattner D. W., Warshaw A. L. Acute hypercalcemia causes acute pancreatitis and ectopic trypsinogen activation in the rat. Gastroenterology. 1995 Jul;109(1):239–246. doi: 10.1016/0016-5085(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Nishimori I., Kamakura M., Fujikawa-Adachi K., Morita M., Onishi S., Yokoyama K., Makino I., Ishida H., Yamamoto M., Watanabe S. Mutations in exons 2 and 3 of the cationic trypsinogen gene in Japanese families with hereditary pancreatitis. Gut. 1999 Feb;44(2):259–263. doi: 10.1136/gut.44.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyaruhucha C. N., Kito M., Fukuoka S. I. Identification and expression of the cDNA-encoding human mesotrypsin(ogen), an isoform of trypsin with inhibitor resistance. J Biol Chem. 1997 Apr 18;272(16):10573–10578. doi: 10.1074/jbc.272.16.10573. [DOI] [PubMed] [Google Scholar]
- Pandya A., Blanton S. H., Landa B., Javaheri R., Melvin E., Nance W. E., Markello T. Linkage studies in a large kindred with hereditary pancreatitis confirms mapping of the gene to a 16-cM region on 7q. Genomics. 1996 Dec 1;38(2):227–230. doi: 10.1006/geno.1996.0620. [DOI] [PubMed] [Google Scholar]
- Perrault J. Hereditary pancreatitis. Gastroenterol Clin North Am. 1994 Dec;23(4):743–752. [PubMed] [Google Scholar]
- Rao K. N., Tuma J., Lombardi B. Acute hemorrhagic pancreatic necrosis in mice. Intraparenchymal activation of zymogens, and other enzyme changes in pancreas and serum. Gastroenterology. 1976 May;70(5 PT1):720–726. [PubMed] [Google Scholar]
- Rinderknecht H. Activation of pancreatic zymogens. Normal activation, premature intrapancreatic activation, protective mechanisms against inappropriate activation. Dig Dis Sci. 1986 Mar;31(3):314–321. doi: 10.1007/BF01318124. [DOI] [PubMed] [Google Scholar]
- Rowen L., Koop B. F., Hood L. The complete 685-kilobase DNA sequence of the human beta T cell receptor locus. Science. 1996 Jun 21;272(5269):1755–1762. doi: 10.1126/science.272.5269.1755. [DOI] [PubMed] [Google Scholar]
- Sarles H., Camarena-Trabous J., Gomez-Santana C., Choux R., Iovanna J. Acute pancreatitis is not a cause of chronic pancreatitis in the absence of residual duct strictures. Pancreas. 1993 May;8(3):354–357. doi: 10.1097/00006676-199305000-00011. [DOI] [PubMed] [Google Scholar]
- Sarles H. Definitions and classifications of pancreatitis. Pancreas. 1991 Jul;6(4):470–474. doi: 10.1097/00006676-199107000-00015. [DOI] [PubMed] [Google Scholar]
- Sibert J. R. Hereditary pancreatitis in England and Wales. J Med Genet. 1978 Jun;15(3):189–201. doi: 10.1136/jmg.15.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossenheimer M. J., Aston C. E., Preston R. A., Gates L. K., Jr, Ulrich C. D., Martin S. P., Zhang Y., Gorry M. C., Ehrlich G. D., Whitcomb D. C. Clinical characteristics of hereditary pancreatitis in a large family, based on high-risk haplotype. The Midwest Multicenter Pancreatic Study Group (MMPSG) Am J Gastroenterol. 1997 Jul;92(7):1113–1116. [PubMed] [Google Scholar]
- Steer M. L., Meldolesi J., Figarella C. Pancreatitis. The role of lysosomes. Dig Dis Sci. 1984 Oct;29(10):934–938. doi: 10.1007/BF01312483. [DOI] [PubMed] [Google Scholar]
- Steinberg W. M., Schlesselman S. E. Treatment of acute pancreatitis. Comparison of animal and human studies. Gastroenterology. 1987 Dec;93(6):1420–1427. doi: 10.1016/0016-5085(87)90275-7. [DOI] [PubMed] [Google Scholar]
- Synn A. Y., Mulvihill S. J., Fonkalsrud E. W. Surgical management of pancreatitis in childhood. J Pediatr Surg. 1987 Jul;22(7):628–632. doi: 10.1016/s0022-3468(87)80114-8. [DOI] [PubMed] [Google Scholar]
- Teich N., Mössner J., Keim V. Mutations of the cationic trypsinogen in hereditary pancreatitis. Hum Mutat. 1998;12(1):39–43. doi: 10.1002/(SICI)1098-1004(1998)12:1<39::AID-HUMU6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Tomita N., Izumoto Y., Horii A., Doi S., Yokouchi H., Ogawa M., Mori T., Matsubara K. Molecular cloning and nucleotide sequence of human pancreatic prechymotrypsinogen cDNA. Biochem Biophys Res Commun. 1989 Jan 31;158(2):569–575. doi: 10.1016/s0006-291x(89)80087-7. [DOI] [PubMed] [Google Scholar]
- Tsui L. C., Durie P. Genotype and phenotype in cystic fibrosis. Hosp Pract (1995) 1997 Jun 15;32(6):115-8, 123-9, 134, passim. doi: 10.1080/21548331.1997.11443512. [DOI] [PubMed] [Google Scholar]
- Vidaud D., Emmerich J., Alhenc-Gelas M., Yvart J., Fiessinger J. N., Aiach M. Met 358 to Arg mutation of alpha 1-antitrypsin associated with protein C deficiency in a patient with mild bleeding tendency. J Clin Invest. 1992 May;89(5):1537–1543. doi: 10.1172/JCI115746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Várallyay E., Pál G., Patthy A., Szilágyi L., Gráf L. Two mutations in rat trypsin confer resistance against autolysis. Biochem Biophys Res Commun. 1998 Feb 4;243(1):56–60. doi: 10.1006/bbrc.1997.8058. [DOI] [PubMed] [Google Scholar]
- Ward J. B., Petersen O. H., Jenkins S. A., Sutton R. Is an elevated concentration of acinar cytosolic free ionised calcium the trigger for acute pancreatitis? Lancet. 1995 Oct 14;346(8981):1016–1019. doi: 10.1016/s0140-6736(95)91695-4. [DOI] [PubMed] [Google Scholar]
- Whitcomb D. C. Genes means pancreatitis. Gut. 1999 Feb;44(2):150–151. doi: 10.1136/gut.44.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcomb D. C., Gorry M. C., Preston R. A., Furey W., Sossenheimer M. J., Ulrich C. D., Martin S. P., Gates L. K., Jr, Amann S. T., Toskes P. P. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996 Oct;14(2):141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- Whitcomb D. C., Preston R. A., Aston C. E., Sossenheimer M. J., Barua P. S., Zhang Y., Wong-Chong A., White G. J., Wood P. G., Gates L. K., Jr A gene for hereditary pancreatitis maps to chromosome 7q35. Gastroenterology. 1996 Jun;110(6):1975–1980. doi: 10.1053/gast.1996.v110.pm8964426. [DOI] [PubMed] [Google Scholar]
- Whitcomb D. C. The First International Symposium on Hereditary Pancreatitis. Pancreas. 1999 Jan;18(1):1–12. [PubMed] [Google Scholar]
- Whitcomb DC. Early trypsinogen activation in acute pancreatitis . Gastroenterology. 1999 Mar;116(3):770–772. [PubMed] [Google Scholar]
- van Laethem J. L., Deviere J., Resibois A., Rickaert F., Vertongen P., Ohtani H., Cremer M., Miyazono K., Robberecht P. Localization of transforming growth factor beta 1 and its latent binding protein in human chronic pancreatitis. Gastroenterology. 1995 Jun;108(6):1873–1881. doi: 10.1016/0016-5085(95)90152-3. [DOI] [PubMed] [Google Scholar]