Abstract
BACKGROUND—Despite intensive research into the molecular abnormalities associated with colorectal cancer (CRC), no diagnostic tests have emerged which usefully complement standard histopathological assessments. AIMS—To assess the feasibility of using immunohistochemistry to detect replication error (RER) positive CRCs and determine the incidence of RER positivity within distinct patient subgroups. METHODS—502 CRCs were analysed for RER positivity (at least two markers affected) and/or expression of hMSH2 and hMLH1. RESULTS—There were 15/30 (50%) patients with metachronous CRCs, 16/51 (31%) with synchronous CRCs, 14/45 (31%) with a proximal colon carcinoma, and 4/23 (17%) who developed a CRC under the age of 50 showed RER positivity. However, 0/54 patients who developed a solitary carcinoma of the rectum/left colon over the age of 50 showed RER positivity. Immunohistochemical analysis revealed that 66/66 (100%) RER positive carcinomas were associated with complete lack of expression of either hMSH2 or hMLH1. This correlation was confirmed using a further 101 proximal colon carcinomas. Patients with a mismatch repair defective carcinoma showed improved survival but a 5.54 times relative risk of developing a metachronous CRC. A prospective immunohistochemical study revealed 13/117 (11%) patients had a mismatch repair defective carcinoma. A fivefold excess of hMLH1 defective cases was noted. CONCLUSIONS—All RER positive carcinomas were identified by the immunohistochemical test. This is the first simple laboratory test which can be performed routinely on all CRCs. It will provide a method for selecting patients who should be investigated for HNPCC, offered long term follow up, and who may not respond to standard chemotherapy regimens. Keywords: colorectal cancer; mismatch repair; hMSH2; hMLH1; microsatellite instability; immunohistochemistry
Full Text
The Full Text of this article is available as a PDF (132.9 KB).
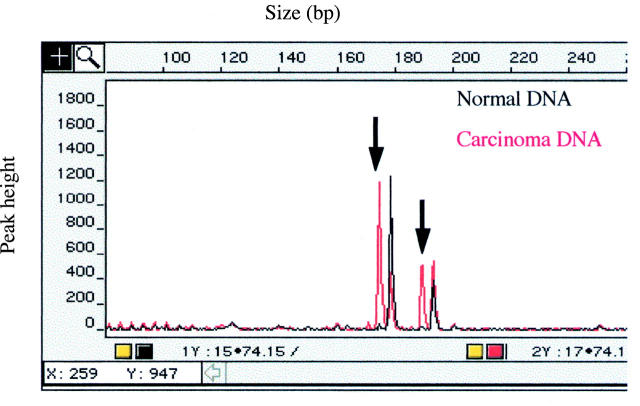
Figure 1 .
Detection of microsatellite instability using the MycL1 marker and the fluorescent PCR based assay. On the cross sectional representation the alleles from the carcinoma DNA and the corresponding normal DNA have been superimposed. The constitutional alleles, which are common to both DNA samples, are colocalised and the novel alleles in the carcinoma DNA (arrowed) are clearly evident.
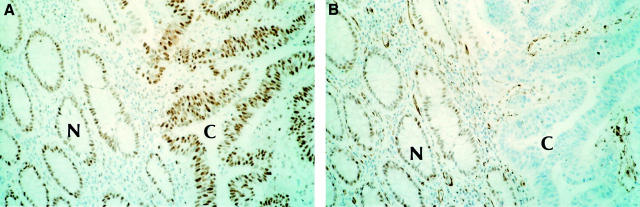
Figure 2 .
Immunohistochemical staining of hMSH2 and hMLH1 using consecutive tissue sections (original magnification × 160). (A) Positive staining of normal control mucosa (N) and adjacent carcinoma (C) with hMSH2. (B) Positive staining of normal control mucosa (N); loss of hMLH1 protein expression in the adjacent carcinoma (C) is clearly evident. Intervening stromal cells are positive.
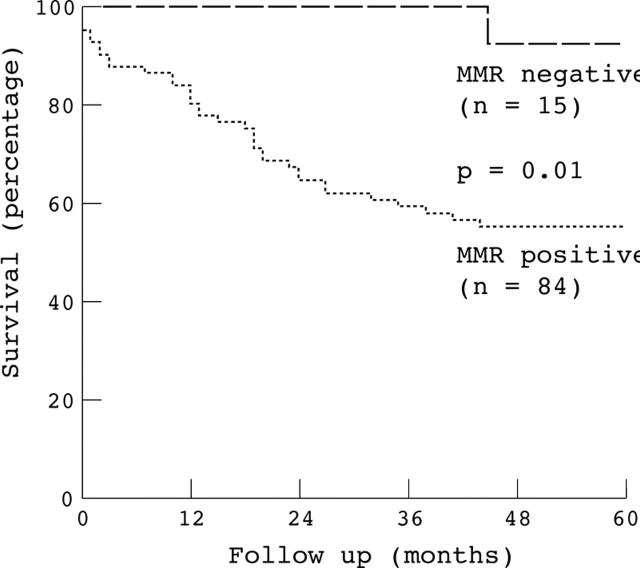
Figure 3 .
Kaplan-Meier survival curve for mismatch repair status, as defined by the expression of hMSH2 and hMLH1, for proximally located sporadic colon carcinomas. A significantly increased patient survival (p=0.01) was associated with the 15 mismatch repair defective (MMR negative) cases (14 lacking hMLH1, one lacking hMSH2) versus the 84 mismatch repair proficient (MMR positive) cases.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Boland C. R. Roles of the DNA mismatch repair genes in colorectal tumorigenesis. Int J Cancer. 1996 Feb 20;69(1):47–49. doi: 10.1002/(SICI)1097-0215(19960220)69:1<47::AID-IJC11>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Branch P., Hampson R., Karran P. DNA mismatch binding defects, DNA damage tolerance, and mutator phenotypes in human colorectal carcinoma cell lines. Cancer Res. 1995 Jun 1;55(11):2304–2309. [PubMed] [Google Scholar]
- Bubb V. J., Curtis L. J., Cunningham C., Dunlop M. G., Carothers A. D., Morris R. G., White S., Bird C. C., Wyllie A. H. Microsatellite instability and the role of hMSH2 in sporadic colorectalcancer. Oncogene. 1996 Jun 20;12(12):2641–2649. [PubMed] [Google Scholar]
- Cawkwell L., Lewis F. A., Quirke P. Frequency of allele loss of DCC, p53, RBI, WT1, NF1, NM23 and APC/MCC in colorectal cancer assayed by fluorescent multiplex polymerase chain reaction. Br J Cancer. 1994 Nov;70(5):813–818. doi: 10.1038/bjc.1994.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawkwell L., Li D., Lewis F. A., Martin I., Dixon M. F., Quirke P. Microsatellite instability in colorectal cancer: improved assessment using fluorescent polymerase chain reaction. Gastroenterology. 1995 Aug;109(2):465–471. doi: 10.1016/0016-5085(95)90334-8. [DOI] [PubMed] [Google Scholar]
- Dietmaier W., Wallinger S., Bocker T., Kullmann F., Fishel R., Rüschoff J. Diagnostic microsatellite instability: definition and correlation with mismatch repair protein expression. Cancer Res. 1997 Nov 1;57(21):4749–4756. [PubMed] [Google Scholar]
- Fante R., Roncucci L., Di GregorioC, Tamassia M. G., Losi L., Benatti P., Pedroni M., Percesepe A., De Pietri S., Ponz de Leon M. Frequency and clinical features of multiple tumors of the large bowel in the general population and in patients with hereditary colorectal carcinoma. Cancer. 1996 May 15;77(10):2013–2021. doi: 10.1002/(SICI)1097-0142(19960515)77:10<2013::AID-CNCR8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Herman J. G., Umar A., Polyak K., Graff J. R., Ahuja N., Issa J. P., Markowitz S., Willson J. K., Hamilton S. R., Kinzler K. W. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci U S A. 1998 Jun 9;95(12):6870–6875. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane M. F., Loda M., Gaida G. M., Lipman J., Mishra R., Goldman H., Jessup J. M., Kolodner R. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997 Mar 1;57(5):808–811. [PubMed] [Google Scholar]
- Liu B., Farrington S. M., Petersen G. M., Hamilton S. R., Parsons R., Papadopoulos N., Fujiwara T., Jen J., Kinzler K. W., Wyllie A. H. Genetic instability occurs in the majority of young patients with colorectal cancer. Nat Med. 1995 Apr;1(4):348–352. doi: 10.1038/nm0495-348. [DOI] [PubMed] [Google Scholar]
- Liu B., Parsons R., Papadopoulos N., Nicolaides N. C., Lynch H. T., Watson P., Jass J. R., Dunlop M., Wyllie A., Peltomäki P. Analysis of mismatch repair genes in hereditary non-polyposis colorectal cancer patients. Nat Med. 1996 Feb;2(2):169–174. doi: 10.1038/nm0296-169. [DOI] [PubMed] [Google Scholar]
- Lothe R. A., Peltomäki P., Meling G. I., Aaltonen L. A., Nyström-Lahti M., Pylkkänen L., Heimdal K., Andersen T. I., Møller P., Rognum T. O. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res. 1993 Dec 15;53(24):5849–5852. [PubMed] [Google Scholar]
- Lynch H. T., Smyrk T., Lynch J. F. Overview of natural history, pathology, molecular genetics and management of HNPCC (Lynch Syndrome). Int J Cancer. 1996 Feb 20;69(1):38–43. doi: 10.1002/(SICI)1097-0215(19960220)69:1<38::AID-IJC9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Smyrk T., Lynch J. An update of HNPCC (Lynch syndrome). Cancer Genet Cytogenet. 1997 Jan;93(1):84–99. doi: 10.1016/s0165-4608(96)00290-7. [DOI] [PubMed] [Google Scholar]
- Mee A. P., Denton J., Hoyland J. A., Davies M., Mawer E. B. Quantification of vitamin D receptor mRNA in tissue sections demonstrates the relative limitations of in situ-reverse transcriptase-polymerase chain reaction. J Pathol. 1997 May;182(1):22–28. doi: 10.1002/(SICI)1096-9896(199705)182:1<22::AID-PATH809>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Mee A. P., Denton J., Hoyland J. A., Davies M., Mawer E. B. Quantification of vitamin D receptor mRNA in tissue sections demonstrates the relative limitations of in situ-reverse transcriptase-polymerase chain reaction. J Pathol. 1997 May;182(1):22–28. doi: 10.1002/(SICI)1096-9896(199705)182:1<22::AID-PATH809>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Mee A. P., Denton J., Hoyland J. A., Davies M., Mawer E. B. Quantification of vitamin D receptor mRNA in tissue sections demonstrates the relative limitations of in situ-reverse transcriptase-polymerase chain reaction. J Pathol. 1997 May;182(1):22–28. doi: 10.1002/(SICI)1096-9896(199705)182:1<22::AID-PATH809>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Mäkelä T. P., Hellsten E., Vesa J., Alitalo K., Peltonen L. An Alu variable polyA repeat polymorphism upstream of L-myc at 1p32. Hum Mol Genet. 1992 Jun;1(3):217–217. doi: 10.1093/hmg/1.3.217-a. [DOI] [PubMed] [Google Scholar]
- Sankila R., Aaltonen L. A., Järvinen H. J., Mecklin J. P. Better survival rates in patients with MLH1-associated hereditary colorectal cancer. Gastroenterology. 1996 Mar;110(3):682–687. doi: 10.1053/gast.1996.v110.pm8608876. [DOI] [PubMed] [Google Scholar]
- Sengupta S. B., Yiu C. Y., Boulos P. B., De Silva M., Sams V. R., Delhanty J. D. Genetic instability in patients with metachronous colorectal cancers. Br J Surg. 1997 Jul;84(7):996–1000. doi: 10.1002/bjs.1800840725. [DOI] [PubMed] [Google Scholar]
- Spirio L., Nelson L., Ward K., Burt R., White R., Leppert M. A CA-repeat polymorphism close to the adenomatous polyposis coli (APC) gene offers improved diagnostic testing for familial APC. Am J Hum Genet. 1993 Feb;52(2):286–296. [PMC free article] [PubMed] [Google Scholar]
- Thibodeau S. N., Bren G., Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993 May 7;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- Thibodeau S. N., French A. J., Cunningham J. M., Tester D., Burgart L. J., Roche P. C., McDonnell S. K., Schaid D. J., Vockley C. W., Michels V. V. Microsatellite instability in colorectal cancer: different mutator phenotypes and the principal involvement of hMLH1. Cancer Res. 1998 Apr 15;58(8):1713–1718. [PubMed] [Google Scholar]
- Thibodeau S. N., French A. J., Roche P. C., Cunningham J. M., Tester D. J., Lindor N. M., Moslein G., Baker S. M., Liskay R. M., Burgart L. J. Altered expression of hMSH2 and hMLH1 in tumors with microsatellite instability and genetic alterations in mismatch repair genes. Cancer Res. 1996 Nov 1;56(21):4836–4840. [PubMed] [Google Scholar]