Abstract
BACKGROUND—Cholera toxin, and Escherichia coli heat labile (LT) and heat stable (STa) enterotoxins induce small intestinal secretion in part by activating enteric nerves. Igmesine is a novel sigma receptor ligand that inhibits neurally mediated secretion. AIMS—To assess the antisecretory potential of igmesine in cholera toxin, LT, and STa induced water and electrolyte secretion using an in vivo rat model of jejunal perfusion. METHODS—After pretreatment with igmesine, 0.03-10 mg/kg intravenously, jejunal segments of anaesthetised, adult male Wistar rats were incubated with cholera toxin (25 µg), LT (25 µg), or saline. Jejunal perfusion with a plasma electrolyte solution containing a non-absorbable marker was undertaken. In some cases 200 µg/l STa was added to the perfusate. After equilibration, net water and electrolyte movement was determined. In additional experiments rats received igmesine, intravenously or intrajejunally, after exposure to cholera toxin. RESULTS—Cholera toxin induced net water secretion was inhibited by 1 mg/kg igmesine (median −120 versus −31 µl/min/g, p<0.001). LT and STa induced secretion were also inhibited by 1 mg/kg igmesine (−90 versus −56, p<0.03; and −76 versus −29, p<0.01, respectively). Igmesine reduced established cholera toxin induced secretion. CONCLUSION—The sigma ligand, igmesine, inhibits neurally mediated enterotoxigenic secretion. Its ability to inhibit established secretion makes it an agent with therapeutic potential. Keywords: igmesine; cholera toxin; Escherichia coli enterotoxin; jejunal secretion
Full Text
The Full Text of this article is available as a PDF (100.7 KB).
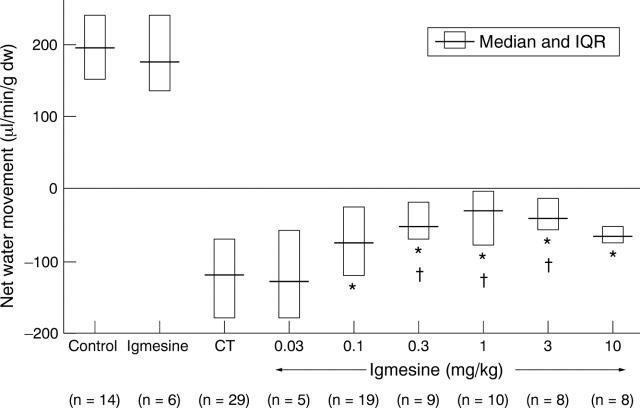
Figure 1 .
Effect of pretreatment with igmesine on cholera toxin (CT) induced jejunal net water secretion. Data expressed as median and interquartile range. *p<0.04 compared with CT; †p<0.02 compared with CT + 0.03 mg/kg igmesine (Kruskal-Wallis).
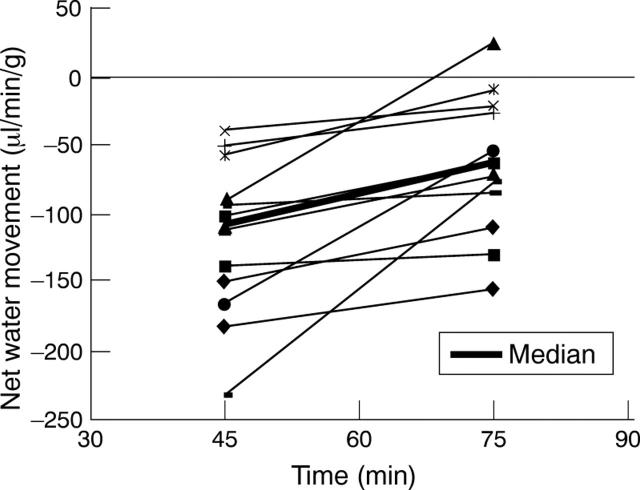
Figure 2 .
Effect of igmesine (1 mg/kg intravenously) on established cholera toxin induced jejunal net water secretion. Net water secretion in individual rats (n=12) is represented at 45 minutes (pre-igmesine) and at 75 minutes (post-igmesine). p<0.04 compared with cholera toxin alone (Mann-Whitney test).
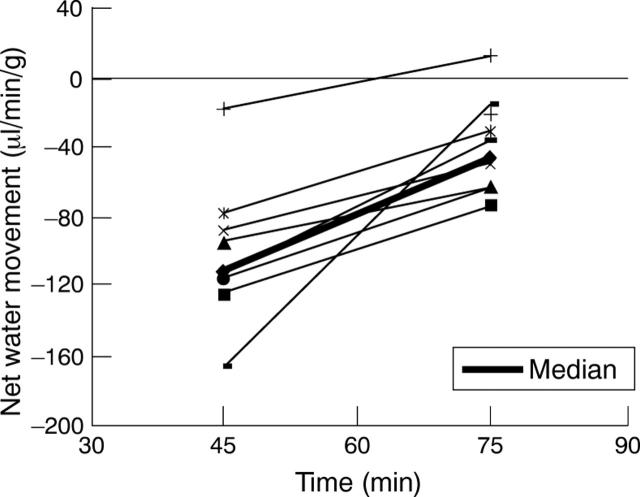
Figure 3 .
Effect of the addition of igmesine (40 µg/ml) to the perfusate, on established cholera toxin induced jejunal net water secretion. Net water secretion in individual rats (n=8) is represented on equilibration at 45 minutes and after receiving 6 mg/kg igmesine, intrajejunally, at 75 minutes. p<0.002 compared with cholera toxin perfused with standard plasma electrolyte solution (Mann-Whitney test).
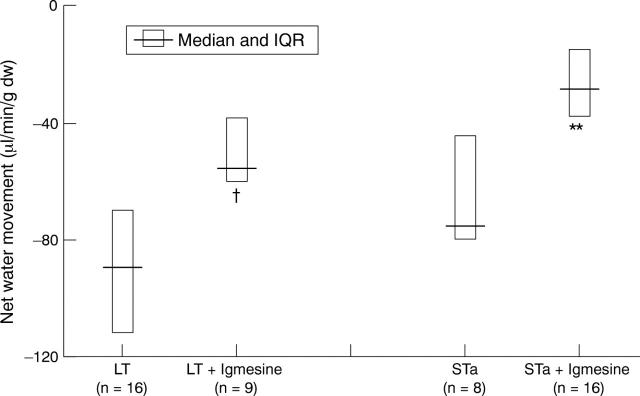
Figure 4 .
Effect of pretreatment with igmesine (1 mg/kg intravenously) on heat labile (LT) and heat stable (STa) enterotoxin induced jejunal net water secretion. Data expressed as median and interquartile range. †p<0.03 compared with LT; **p<0.01 compared with STa (Mann-Whitney test).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beubler E., Horina G. 5-HT2 and 5-HT3 receptor subtypes mediate cholera toxin-induced intestinal fluid secretion in the rat. Gastroenterology. 1990 Jul;99(1):83–89. doi: 10.1016/0016-5085(90)91233-v. [DOI] [PubMed] [Google Scholar]
- Beubler E., Kollar G., Saria A., Bukhave K., Rask-Madsen J. Involvement of 5-hydroxytryptamine, prostaglandin E2, and cyclic adenosine monophosphate in cholera toxin-induced fluid secretion in the small intestine of the rat in vivo. Gastroenterology. 1989 Feb;96(2 Pt 1):368–376. doi: 10.1016/0016-5085(89)91560-6. [DOI] [PubMed] [Google Scholar]
- Campbell B. G., Scherz M. W., Keana J. F., Weber E. Sigma receptors regulate contractions of the guinea pig ileum longitudinal muscle/myenteric plexus preparation elicited by both electrical stimulation and exogenous serotonin. J Neurosci. 1989 Oct;9(10):3380–3391. doi: 10.1523/JNEUROSCI.09-10-03380.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3307–3311. doi: 10.1073/pnas.74.8.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassuto J., Fahrenkrug J., Jodal M., Tuttle R., Lundgren O. Release of vasoactive intestinal polypeptide from the cat small intestine exposed to cholera toxin. Gut. 1981 Nov;22(11):958–963. doi: 10.1136/gut.22.11.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M., Binder H. J. Effect of enterotoxins of Vibrio cholerae, Escherichia coli, and Shigella dysenteriae type 1 on fluid and electrolyte transport in the colon. J Infect Dis. 1976 Aug;134(2):135–143. doi: 10.1093/infdis/134.2.135. [DOI] [PubMed] [Google Scholar]
- Elliott E. J., Watson A. J., Walker-Smith J. A., Farthing M. J. Search for the ideal oral rehydration solution: studies in a model of secretory diarrhoea. Gut. 1991 Nov;32(11):1314–1320. doi: 10.1136/gut.32.11.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M. Modes of action of enterotoxins from Vibrio cholerae and EScherichia coli. Rev Infect Dis. 1979 Nov-Dec;1(6):918–926. doi: 10.1093/clinids/1.6.918. [DOI] [PubMed] [Google Scholar]
- Field M., Rao M. C., Chang E. B. Intestinal electrolyte transport and diarrheal disease (1). N Engl J Med. 1989 Sep 21;321(12):800–806. doi: 10.1056/NEJM198909213211206. [DOI] [PubMed] [Google Scholar]
- Gotuzzo E., Seas C., Echevarría J., Carrillo C., Mostorino R., Ruiz R. Ciprofloxacin for the treatment of cholera: a randomized, double-blind, controlled clinical trial of a single daily dose in Peruvian adults. Clin Infect Dis. 1995 Jun;20(6):1485–1490. doi: 10.1093/clinids/20.6.1485. [DOI] [PubMed] [Google Scholar]
- Griffiths S. L., Critchley D. R. Characterisation of the binding sites for Escherichia coli heat-labile toxin type I in intestinal brush borders. Biochim Biophys Acta. 1991 Oct 10;1075(2):154–161. doi: 10.1016/0304-4165(91)90246-d. [DOI] [PubMed] [Google Scholar]
- Guarino A., Cohen M., Thompson M., Dharmsathaphorn K., Giannella R. T84 cell receptor binding and guanyl cyclase activation by Escherichia coli heat-stable toxin. Am J Physiol. 1987 Dec;253(6 Pt 1):G775–G780. doi: 10.1152/ajpgi.1987.253.6.G775. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Fredman P., Lindblad M., Svennerholm A. M., Svennerholm L. Rabbit intestinal glycoprotein receptor for Escherichia coli heat-labile enterotoxin lacking affinity for cholera toxin. Infect Immun. 1982 Nov;38(2):424–433. doi: 10.1128/iai.38.2.424-433.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren O., Svanvik J., Jivegård L. Enteric nervous system. I. Physiology and pathophysiology of the intestinal tract. Dig Dis Sci. 1989 Feb;34(2):264–283. doi: 10.1007/BF01536062. [DOI] [PubMed] [Google Scholar]
- Martin W. R., Eades C. G., Thompson J. A., Huppler R. E., Gilbert P. E. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976 Jun;197(3):517–532. [PubMed] [Google Scholar]
- Mourad F. H., O'Donnell L. J., Dias J. A., Ogutu E., Andre E. A., Turvill J. L., Farthing M. J. Role of 5-hydroxytryptamine type 3 receptors in rat intestinal fluid and electrolyte secretion induced by cholera and Escherichia coli enterotoxins. Gut. 1995 Sep;37(3):340–345. doi: 10.1136/gut.37.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson O., Cassuto J., Larsson P. A., Jodal M., Lidberg P., Ahlman H., Dahlström A., Lundgren O. 5-Hydroxytryptamine and cholera secretion: a histochemical and physiological study in cats. Gut. 1983 Jun;24(6):542–548. doi: 10.1136/gut.24.6.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascaud X. B., Chovet M., Roze C., Junien J. L. Neuropeptide Y and sigma receptor agonists act through a common pathway to stimulate duodenal alkaline secretion in rats. Eur J Pharmacol. 1993 Feb 16;231(3):389–394. doi: 10.1016/0014-2999(93)90115-x. [DOI] [PubMed] [Google Scholar]
- Riviere P. J., Rao R. K., Pascaud X., Junien J. L., Porreca F. Effects of neuropeptide Y, peptide YY and sigma ligands on ion transport in mouse jejunum. J Pharmacol Exp Ther. 1993 Mar;264(3):1268–1274. [PubMed] [Google Scholar]
- Rivière P. J., Pascaud X., Junien J. L., Porreca F. Neuropeptide Y and JO 1784, a selective sigma ligand, alter intestinal ion transport through a common, haloperidol-sensitive site. Eur J Pharmacol. 1990 Oct 23;187(3):557–559. doi: 10.1016/0014-2999(90)90388-m. [DOI] [PubMed] [Google Scholar]
- Rolston D. D., Borodo M. M., Kelly M. J., Dawson A. M., Farthing M. J. Efficacy of oral rehydration solutions in a rat model of secretory diarrhea. J Pediatr Gastroenterol Nutr. 1987 Jul-Aug;6(4):624–630. doi: 10.1097/00005176-198707000-00023. [DOI] [PubMed] [Google Scholar]
- Roman F. J., Pascaud X., Duffy O., Vauche D., Martin B., Junien J. L. Neuropeptide Y and peptide YY interact with rat brain sigma and PCP binding sites. Eur J Pharmacol. 1989 Dec 19;174(2-3):301–302. doi: 10.1016/0014-2999(89)90326-9. [DOI] [PubMed] [Google Scholar]
- Roman F. J., Pascaud X., Martin B., Vauché D., Junien J. L. JO 1784, a potent and selective ligand for rat and mouse brain sigma-sites. J Pharm Pharmacol. 1990 Jun;42(6):439–440. doi: 10.1111/j.2042-7158.1990.tb06588.x. [DOI] [PubMed] [Google Scholar]
- Roman F., Pascaud X., Chomette G., Bueno L., Junien J. L. Autoradiographic localization of sigma opioid receptors in the gastrointestinal tract of the guinea pig. Gastroenterology. 1989 Jul;97(1):76–82. doi: 10.1016/0016-5085(89)91418-2. [DOI] [PubMed] [Google Scholar]
- Roman F., Pascaud X., Vauché D., Junien J. L. Evidence for a non-opioid sigma binding site in the guinea-pig myenteric plexus. Life Sci. 1988;42(22):2217–2222. doi: 10.1016/0024-3205(88)90373-6. [DOI] [PubMed] [Google Scholar]
- Rozé C., Bruley Des Varannes S., Shi G., Genéve J., Galmiche J. P. Inhibition of prostaglandin-induced intestinal secretion by igmesine in healthy volunteers. Gastroenterology. 1998 Sep;115(3):591–596. doi: 10.1016/s0016-5085(98)70138-6. [DOI] [PubMed] [Google Scholar]
- Sjöqvist A., Fahrenkrug J., Jodal M., Lundgren O. The effect of splanchnic nerve stimulation and neuropeptide Y on cholera secretion and release of vasoactive intestinal polypeptide in the feline small intestine. Acta Physiol Scand. 1988 Jul;133(3):289–295. doi: 10.1111/j.1748-1716.1988.tb08410.x. [DOI] [PubMed] [Google Scholar]
- Souli A., Chariot J., Presset O., Tsocas A., Rozé C. Neural modulation of the antisecretory effect of peptide YY in the rat jejunum. Eur J Pharmacol. 1997 Aug 20;333(1):87–92. doi: 10.1016/s0014-2999(97)01112-6. [DOI] [PubMed] [Google Scholar]
- Spangler B. D. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992 Dec;56(4):622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam S. W., Mitchell K. N. Neuropeptide Y and peptide YY do not bind to brain sigma and phencyclidine binding sites. Eur J Pharmacol. 1991 Jan 25;193(1):121–122. doi: 10.1016/0014-2999(91)90210-h. [DOI] [PubMed] [Google Scholar]
- Tantisira M. H., Jodal M., Lundgren O. Further studies of the changes in alkaline secretion, transepithelial potential difference and net fluid transport induced by the heat-stable enterotoxin of Escherichia coli (STa) in the rat jejunum in vivo. Acta Physiol Scand. 1990 Dec;140(4):557–565. doi: 10.1111/j.1748-1716.1990.tb09033.x. [DOI] [PubMed] [Google Scholar]
- Turvill J. L., Mourad F. H., Farthing M. J. Crucial role for 5-HT in cholera toxin but not Escherichia coli heat-labile enterotoxin-intestinal secretion in rats. Gastroenterology. 1998 Oct;115(4):883–890. doi: 10.1016/s0016-5085(98)70260-4. [DOI] [PubMed] [Google Scholar]
- Walker J. M., Bowen W. D., Walker F. O., Matsumoto R. R., De Costa B., Rice K. C. Sigma receptors: biology and function. Pharmacol Rev. 1990 Dec;42(4):355–402. [PubMed] [Google Scholar]
- Zemelman B. V., Chu S. H., Walker W. A. Host response to Escherichia coli heat-labile enterotoxin via two microvillus membrane receptors in the rat intestine. Infect Immun. 1989 Oct;57(10):2947–2952. doi: 10.1128/iai.57.10.2947-2952.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]