Abstract
BACKGROUND—Phospholipase A2 (PLA2) is involved in regulating biosynthesis of arachidonic acid and its metabolites. There are three major structurally different forms of PLA2: group I, also called pancreatic PLA2 (PLA2-I); group II, referred to as secretory non-pancreatic or synovial or platelet PLA2 (PLA2-II); group IV, referred to as cytosolic PLA2 (PLA2-IV). AIMS—To examine PLA2-I, PLA2-II, and PLA2-IV in normal and pancreatic cancer tissues. Patients—PLA2 was studied in 58 pancreatic adenocarcinomas, obtained from 25 women and 33 men undergoing pancreatic resection. Normal organ donor pancreas served as control. METHODS—The enzymes were analysed by northern blot, in situ hybridisation, and immunohistochemistry. The molecular findings were correlated with clinical variables of the patients. RESULTS—Northern blot analysis of total RNA showed enhanced PLA2 group II and IV mRNA expression in 52% and 55% of the pancreatic cancer samples respectively compared with the normal controls (p = 0.0013 and p = 0.0025). On immunohistochemical analysis, intense PLA2-I immunoreactivity was seen in acinar cells, but not in ductal cells, in the normal pancreas. In pancreatic cancer cells, PLA2-I immunostaining was absent. PLA2-II immunostaining was visible only in some acinar and ductal cells in the normal pancreas, whereas in pancreatic cancer increased PLA2-II immunoreactivity was present in 65% of the cancer samples. On in situ hybridisation, weak PLA2-IV mRNA signals were detected in acinar and ductal cells of normal samples; these signals were present to a much greater extent in pancreatic cancer cells. The presence of PLA2-II in pancreatic cancer was associated with a higher degree of fibrosis (p<0.01). Furthermore, there was a significant correlation between the enhanced expression of PLA2-II and longer survival after surgery (p<0.03), but not of PLA2-IV and longer postoperative survival. CONCLUSION—These data suggest that PLA2-II and PLA2-IV are upregulated in human pancreatic cancer, and that upregulation of PLA2-II in pancreatic cancer covariates negatively with cancer cell growth. Keywords: pancreas; cancer; phospholipase A2; survival analysis
Full Text
The Full Text of this article is available as a PDF (186.1 KB).
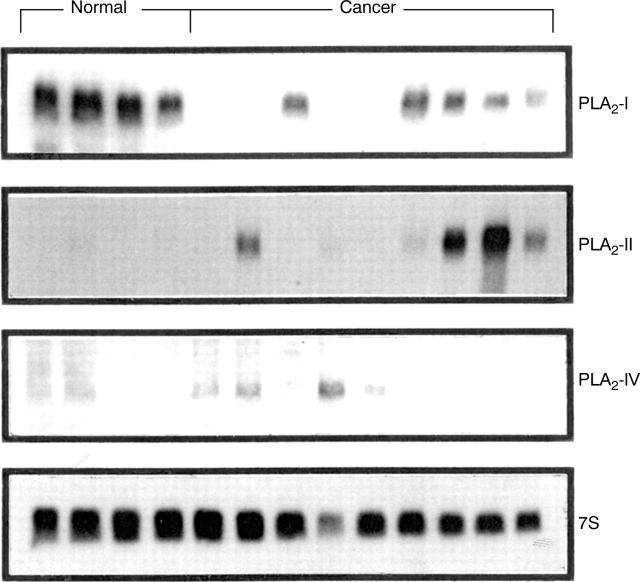
Figure 1 .
Northern blot analysis of phospholipase A2 (PLA2)-I, PLA2-II, and PLA2-IV mRNA in the normal pancreas (first four lanes) and in pancreatic tissues obtained from patients with pancreatic cancer (last nine lanes). In cancer samples, levels of PLA2-I mRNA were reduced compared with the normal controls. In contrast, enhanced levels of PLA2-II and PLA2-IV mRNA were found in many cancer samples, whereas low to undetectable PLA2-II and PLA2-IV mRNA expression was present in normal controls. 7S mRNA was used to assess equivalent RNA loading.
Figure 2 .
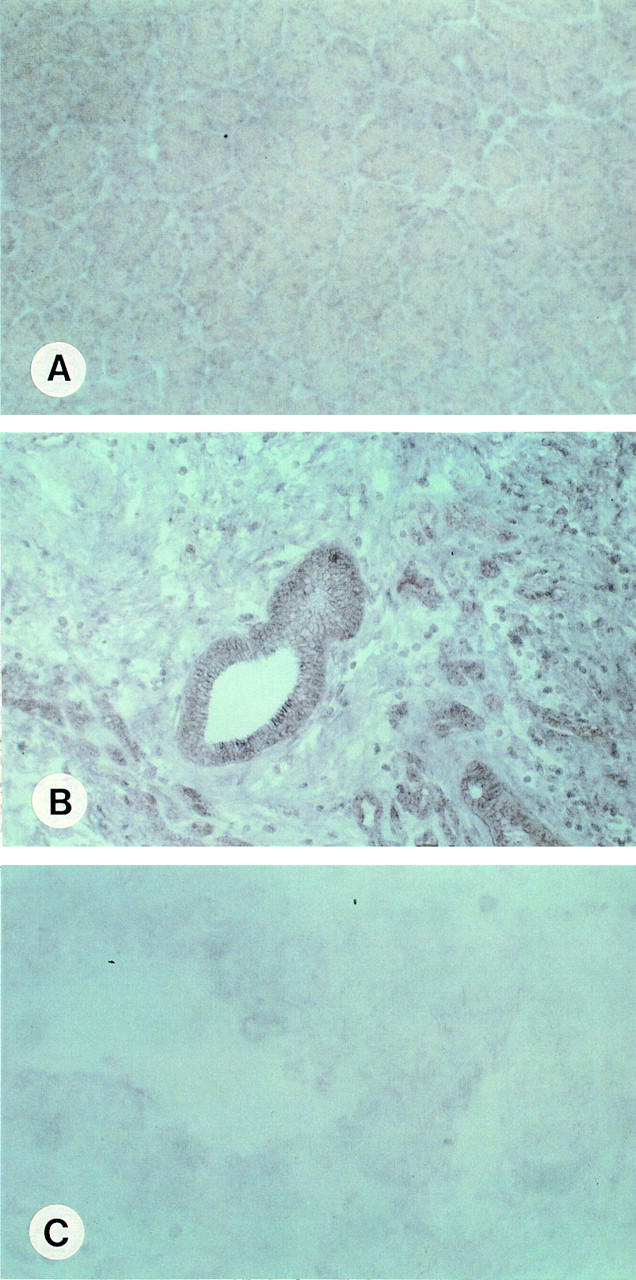
In situ hybridisation of phospholipase A2 (PLA2)-IV in the normal pancreas (A) and in pancreatic cancer (B, antisense hybridisation, C, sense hybridisation). In the normal pancreas, faint PLA2-IV mRNA signals were present in some acinar cells (A). In contrast, intense PLA2-IV mRNA signals were found in the pancreatic cancer cells (B). (C) Sense hybridisation of a pancreatic cancer sample which exhibited high levels of PLA2-IV mRNA in northern blot hybridisation. Original magnifications: A, B × 200; C × 400.
Figure 3 .
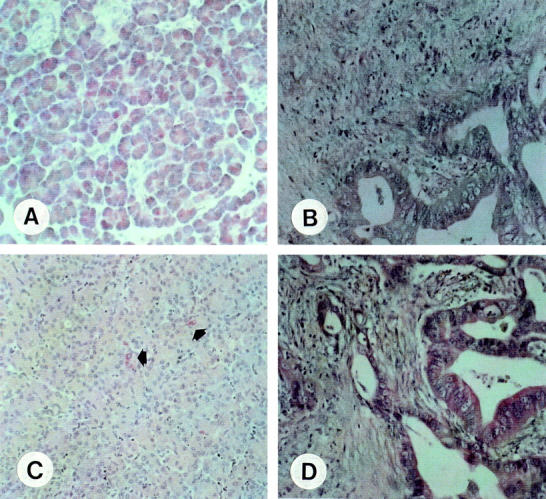
Immunohistochemical analysis of phospholipase A2 (PLA2)-I (A, B) and PLA2-II (C, D) in the normal pancreas (A, C) and in pancreatic cancer (B, D). PLA2-I immunostaining was abundant in acinar cells of the normal pancreas (A). No PLA2-I immunostaining was present in the pancreatic cancer cells (B). In the normal pancreas, only a few acinar cells exhibited cytoplasmic PLA2-II immunoreactivity (black arrows, C), whereas PLA2-II immunostaining was often present in pancreatic cancer cells (D). The slides are counterstained with haematoxylin. Original magnifications × 200.
Figure 4 .
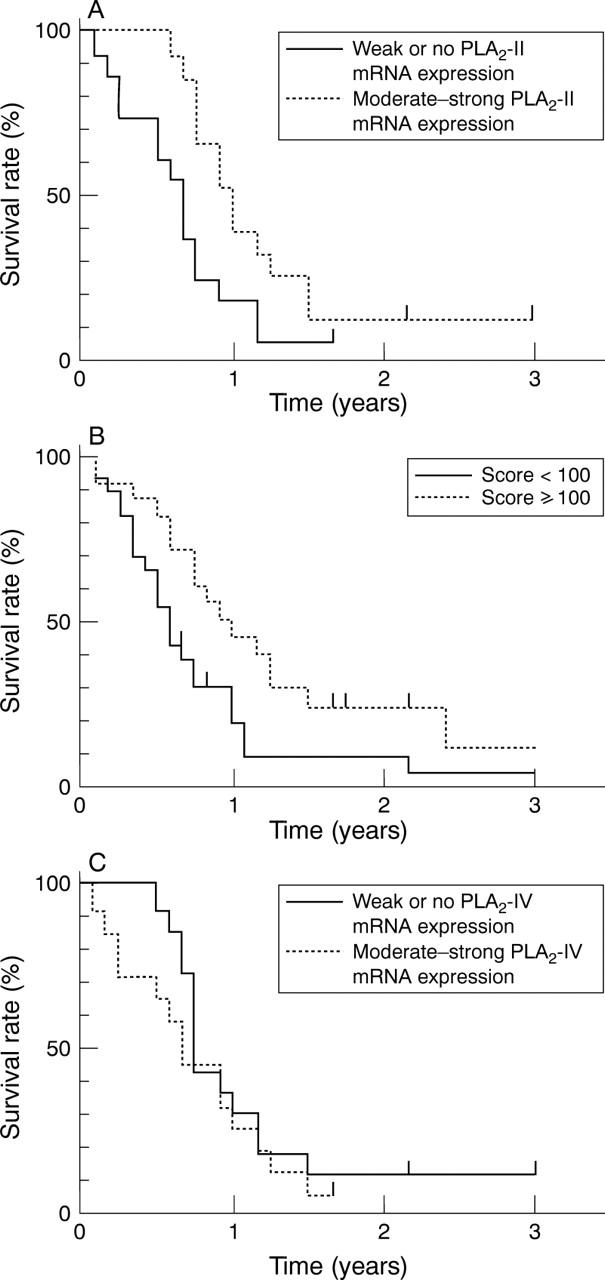
Survival curves. (A) Kaplan-Meier plots of the postoperative survival periods in patients whose tumours were defined as having weak or no phospholipase A2 (PLA2)-II mRNA expression versus patients with moderate to strong PLA2-II mRNA expression (broken line). Cox analysis of the postoperative survival periods showed that patients whose tumours exhibited weak or no PLA2-II mRNA expression lived a significantly shorter time (p<0.02) than those whose tumours exhibited moderate to strong PLA2-II mRNA levels. (B) Kaplan-Meier plots of the postoperative survival periods in patients whose tumours were defined as having a PLA2-II immunoreactivity score <100 versus patients with a PLA2-II immunoreactivity score ⩾100. Cox analysis of the postoperative survival periods showed that patients whose tumours exhibited a low PLA2-II immunoreactivity score lived a significantly shorter time (p<0.05) than those with a higher immunoreactivity score. (C) Kaplan-Meier plots of the postoperative survival periods in patients whose tumours were defined as having weak or no PLA2-IV mRNA expression versus patients with moderate to strong PLA2-IV mRNA expression. Cox analysis of the postoperative survival periods indicated no difference in survival between these two patient groups.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacus S., Flowers J. L., Press M. F., Bacus J. W., McCarty K. S., Jr The evaluation of estrogen receptor in primary breast carcinoma by computer-assisted image analysis. Am J Clin Pathol. 1988 Sep;90(3):233–239. doi: 10.1093/ajcp/90.3.233. [DOI] [PubMed] [Google Scholar]
- Büchler M., Malfertheiner P., Schädlich H., Nevalainen T. J., Friess H., Beger H. G. Role of phospholipase A2 in human acute pancreatitis. Gastroenterology. 1989 Dec;97(6):1521–1526. doi: 10.1016/0016-5085(89)90398-3. [DOI] [PubMed] [Google Scholar]
- Clark J. D., Lin L. L., Kriz R. W., Ramesha C. S., Sultzman L. A., Lin A. Y., Milona N., Knopf J. L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991 Jun 14;65(6):1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- Dennis E. A. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994 May 6;269(18):13057–13060. [PubMed] [Google Scholar]
- Dennis E. A. The growing phospholipase A2 superfamily of signal transduction enzymes. Trends Biochem Sci. 1997 Jan;22(1):1–2. doi: 10.1016/s0968-0004(96)20031-3. [DOI] [PubMed] [Google Scholar]
- Ebert M., Yokoyama M., Friess H., Kobrin M. S., Büchler M. W., Korc M. Induction of platelet-derived growth factor A and B chains and over-expression of their receptors in human pancreatic cancer. Int J Cancer. 1995 Sep 4;62(5):529–535. doi: 10.1002/ijc.2910620507. [DOI] [PubMed] [Google Scholar]
- Friess H., Berberat P., Schilling M., Kunz J., Korc M., Büchler M. W. Pancreatic cancer: the potential clinical relevance of alterations in growth factors and their receptors. J Mol Med (Berl) 1996 Jan;74(1):35–42. doi: 10.1007/BF00202070. [DOI] [PubMed] [Google Scholar]
- Friess H., Cantero D., Graber H., Tang W. H., Guo X., Kashiwagi M., Zimmermann A., Gold L., Korc M., Büchler M. W. Enhanced urokinase plasminogen activation in chronic pancreatitis suggests a role in its pathogenesis. Gastroenterology. 1997 Sep;113(3):904–913. doi: 10.1016/s0016-5085(97)70186-0. [DOI] [PubMed] [Google Scholar]
- Friess H., Malfertheiner P., Isenmann R., Kühne H., Beger H. G., Büchler M. W. The risk of pancreaticointestinal anastomosis can be predicted preoperatively. Pancreas. 1996 Aug;13(2):202–208. [PubMed] [Google Scholar]
- Fulton A. M. The role of eicosanoids in tumor metastasis. Prostaglandins Leukot Essent Fatty Acids. 1988;34(3):229–237. [PubMed] [Google Scholar]
- Goddard D. H., Bomalaski J. S., Lipper S., Shorr R. G., Clark M. A. Phospholipase A2-mediated inflammation induces regression of malignant gliomas. Cancer Lett. 1996 Apr 19;102(1-2):1–6. doi: 10.1016/0304-3835(96)04142-0. [DOI] [PubMed] [Google Scholar]
- Gress T. M., Müller-Pillasch F., Lerch M. M., Friess H., Büchler M., Adler G. Expression and in-situ localization of genes coding for extracellular matrix proteins and extracellular matrix degrading proteases in pancreatic cancer. Int J Cancer. 1995 Aug 9;62(4):407–413. doi: 10.1002/ijc.2910620409. [DOI] [PubMed] [Google Scholar]
- Grünewald K., Lyons J., Fröhlich A., Feichtinger H., Weger R. A., Schwab G., Janssen J. W., Bartram C. R. High frequency of Ki-ras codon 12 mutations in pancreatic adenocarcinomas. Int J Cancer. 1989 Jun 15;43(6):1037–1041. doi: 10.1002/ijc.2910430614. [DOI] [PubMed] [Google Scholar]
- Guo X., Friess H., Graber H. U., Kashiwagi M., Zimmermann A., Korc M., Büchler M. W. KAI1 expression is up-regulated in early pancreatic cancer and decreased in the presence of metastases. Cancer Res. 1996 Nov 1;56(21):4876–4880. [PubMed] [Google Scholar]
- Hanada K., Kinoshita E., Itoh M., Hirata M., Kajiyama G., Sugiyama M. Human pancreatic phospholipase A2 stimulates the growth of human pancreatic cancer cell line. FEBS Lett. 1995 Oct 2;373(1):85–87. doi: 10.1016/0014-5793(95)01005-y. [DOI] [PubMed] [Google Scholar]
- Hara S., Kudo I., Inoue K. Augmentation of prostaglandin E2 production by mammalian phospholipase A2 added exogenously. J Biochem. 1991 Aug;110(2):163–165. doi: 10.1093/oxfordjournals.jbchem.a123550. [DOI] [PubMed] [Google Scholar]
- Heasley L. E., Thaler S., Nicks M., Price B., Skorecki K., Nemenoff R. A. Induction of cytosolic phospholipase A2 by oncogenic Ras in human non-small cell lung cancer. J Biol Chem. 1997 Jun 6;272(23):14501–14504. doi: 10.1074/jbc.272.23.14501. [DOI] [PubMed] [Google Scholar]
- Hendrickse C. W., Radley S., Donovan I. A., Keighley M. R., Neoptolemos J. P. Activities of phospholipase A2 and diacylglycerol lipase are increased in human colorectal cancer. Br J Surg. 1995 Apr;82(4):475–478. doi: 10.1002/bjs.1800820415. [DOI] [PubMed] [Google Scholar]
- Ishizaki J., Ohara O., Nakamura E., Tamaki M., Ono T., Kanda A., Yoshida N., Teraoka H., Tojo H., Okamoto M. cDNA cloning and sequence determination of rat membrane-associated phospholipase A2. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1030–1036. doi: 10.1016/0006-291x(89)90777-8. [DOI] [PubMed] [Google Scholar]
- Kanda A., Ono T., Yoshida N., Tojo H., Okamoto M. The primary structure of a membrane-associated phospholipase A2 from human spleen. Biochem Biophys Res Commun. 1989 Aug 30;163(1):42–48. doi: 10.1016/0006-291x(89)92096-2. [DOI] [PubMed] [Google Scholar]
- Kashiwagi M., Friess H., Uhl W., Graber H., Duarte R., Zimmermann A., Büchler M. W. Phospholipase A2 isoforms are altered in chronic pancreatitis. Ann Surg. 1998 Feb;227(2):220–228. doi: 10.1097/00000658-199802000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyohara H., Egami H., Kako H., Shibata Y., Murata K., Ohshima S., Sei K., Suko S., Kurano R., Ogawa M. Immunohistochemical localization of group II phospholipase A2 in human pancreatic carcinomas. Int J Pancreatol. 1993 Feb;13(1):49–57. doi: 10.1007/BF02795199. [DOI] [PubMed] [Google Scholar]
- Korc M., Chandrasekar B., Yamanaka Y., Friess H., Buchier M., Beger H. G. Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increases in the levels of epidermal growth factor and transforming growth factor alpha. J Clin Invest. 1992 Oct;90(4):1352–1360. doi: 10.1172/JCI116001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. M., Sharp J. D. Recent insights into the structure, function and biology of cPLA2. Agents Actions Suppl. 1995;46:65–76. doi: 10.1007/978-3-0348-7276-8_7. [DOI] [PubMed] [Google Scholar]
- Kudo I., Murakami M., Hara S., Inoue K. Mammalian non-pancreatic phospholipases A2. Biochim Biophys Acta. 1993 Nov 3;1170(3):217–231. doi: 10.1016/0005-2760(93)90003-r. [DOI] [PubMed] [Google Scholar]
- Kurizaki T., Egami H., Murata K., Kiyohara H., Okazaki S., Yoshida N., Ogawa M. Membrane-associated phospholipase A2 stimulates DNA synthesis in two murine fibroblasts. Res Commun Chem Pathol Pharmacol. 1992 Oct;78(1):39–45. [PubMed] [Google Scholar]
- Marnett L. J. Aspirin and the potential role of prostaglandins in colon cancer. Cancer Res. 1992 Oct 15;52(20):5575–5589. [PubMed] [Google Scholar]
- Mayer R. J., Marshall L. A. New insights on mammalian phospholipase A2(s); comparison of arachidonoyl-selective and -nonselective enzymes. FASEB J. 1993 Feb 1;7(2):339–348. doi: 10.1096/fasebj.7.2.8440410. [DOI] [PubMed] [Google Scholar]
- Murata K., Egami H., Kiyohara H., Oshima S., Kurizaki T., Ogawa M. Expression of group-II phospholipase A2 in malignant and non-malignant human gastric mucosa. Br J Cancer. 1993 Jul;68(1):103–111. doi: 10.1038/bjc.1993.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevalainen T. J., Grönroos J. M., Kallajoki M. Expression of group II phospholipase A2 in the human gastrointestinal tract. Lab Invest. 1995 Feb;72(2):201–208. [PubMed] [Google Scholar]
- Nevalainen T. J., Kortesuo P. T., Rintala E., Märki F. Immunochemical detection of group I and group II phospholipases A2 in human serum. Clin Chem. 1992 Sep;38(9):1824–1829. [PubMed] [Google Scholar]
- Nolan R. D., Danilowicz R. M., Eling T. E. Role of arachidonic acid metabolism in the mitogenic response of BALB/c 3T3 fibroblasts to epidermal growth factor. Mol Pharmacol. 1988 Jun;33(6):650–656. [PubMed] [Google Scholar]
- Nomura K., Fujita H., Arita H. Gene expression of pancreatic-type phospholipase-A2 in rat ovaries: stimulatory action on progesterone release. Endocrinology. 1994 Aug;135(2):603–609. doi: 10.1210/endo.135.2.8033809. [DOI] [PubMed] [Google Scholar]
- Ohara O., Ishizaki J., Nakano T., Arita H., Teraoka H. A simple and sensitive method for determining transcription initiation site: identification of two transcription initiation sites in rat group II phospholipase A2 gene. Nucleic Acids Res. 1990 Dec 11;18(23):6997–7002. doi: 10.1093/nar/18.23.6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald E., Tibes U., Maass G., Scheuer W. Induction of cytosolic phospholipase A2 in human leukocytes by lipopolysaccharide. Eur J Biochem. 1994 Aug 1;223(3):743–749. doi: 10.1111/j.1432-1033.1994.tb19048.x. [DOI] [PubMed] [Google Scholar]
- Santavuori S. A., Kortesuo P. T., Eskola J. U., Nevalainen T. J. Application of a new monoclonal antibody for time-resolved fluoroimmunoassay of human pancreatic phospholipase A2. Eur J Clin Chem Clin Biochem. 1991 Dec;29(12):819–826. [PubMed] [Google Scholar]
- Seilhamer J. J., Randall T. L., Yamanaka M., Johnson L. K. Pancreatic phospholipase A2: isolation of the human gene and cDNAs from porcine pancreas and human lung. DNA. 1986 Dec;5(6):519–527. doi: 10.1089/dna.1.1986.5.519. [DOI] [PubMed] [Google Scholar]
- Soydan A. S., Tavares I. A., Weech P. K., Temblay N. M., Bennett A. High molecular weight phospholipase A2 and fatty acids in human colon tumours and associated normal tissue. Eur J Cancer. 1996 Sep;32A(10):1781–1787. doi: 10.1016/0959-8049(96)00166-9. [DOI] [PubMed] [Google Scholar]
- Uhl W., Beger H. G., Hoffmann G., Hanisch E., Schild A., Waydhas C., Entholzner E., Müller K., Kellermann W., Vogeser M. A multicenter study of phospholipase A2 in patients in intensive care units. J Am Coll Surg. 1995 Mar;180(3):323–331. [PubMed] [Google Scholar]
- Uhl W., Schrag H. J., Schmitter N., Aufenanger J., Nevalainen T. J., Büchler M. W. Experimental study of a novel phospholipase A2 inhibitor in acute pancreatitis. Br J Surg. 1998 May;85(5):618–623. doi: 10.1046/j.1365-2168.1998.00674.x. [DOI] [PubMed] [Google Scholar]
- Uhl W., Schrag H. J., Schmitter N., Nevalainen T. J., Aufenanger J., Wheatley A. M., Büchler M. W. Pathophysiological role of secretory type I and II phospholipase A2 in acute pancreatitis: an experimental study in rats. Gut. 1997 Mar;40(3):386–392. doi: 10.1136/gut.40.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadas P., Browning J., Edelson J., Pruzanski W. Extracellular phospholipase A2 expression and inflammation: the relationship with associated disease states. J Lipid Mediat. 1993 Aug;8(1):1–30. [PubMed] [Google Scholar]
- Wickremasinghe R. G. The role of prostaglandins in the regulation of cell proliferation. Prostaglandins Leukot Essent Fatty Acids. 1988;31(3):171–179. [PubMed] [Google Scholar]
- Yamanaka Y., Friess H., Buchler M., Beger H. G., Uchida E., Onda M., Kobrin M. S., Korc M. Overexpression of acidic and basic fibroblast growth factors in human pancreatic cancer correlates with advanced tumor stage. Cancer Res. 1993 Nov 1;53(21):5289–5296. [PubMed] [Google Scholar]
- Yamanaka Y., Friess H., Kobrin M. S., Buchler M., Beger H. G., Korc M. Coexpression of epidermal growth factor receptor and ligands in human pancreatic cancer is associated with enhanced tumor aggressiveness. Anticancer Res. 1993 May-Jun;13(3):565–569. [PubMed] [Google Scholar]
- Yamashita S., Yamashita J., Ogawa M. Overexpression of group II phospholipase A2 in human breast cancer tissues is closely associated with their malignant potency. Br J Cancer. 1994 Jun;69(6):1166–1170. doi: 10.1038/bjc.1994.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z., Tojo H., Komatsubara T., Nakagawa M., Inada M., Kawata S., Matsuzawa Y., Okamoto M. Enhanced expression of group II phospholipase A2 in human hepatocellular carcinoma. Biochim Biophys Acta. 1994 May 25;1226(2):201–205. doi: 10.1016/0925-4439(94)90029-9. [DOI] [PubMed] [Google Scholar]