Abstract
BACKGROUND—Neutrophil-endothelial cell interactions are thought to play a critical role in the pathophysiology of non-steroidal anti-inflammatory drug (NSAID) induced gastropathy. AIMS—To optimise a mouse model of NSAID induced gastropathy and to evaluate the importance of adhesion molecules using adhesion molecule deficient mice. METHODS—Gastropathy was induced in C57BL/6 mice or their adhesion molecule deficient counterparts via oral administration of indomethacin (20 mg/kg). Lesion scores, mucosal permeability, and histopathology were used to assess gastric mucosal injury. RESULTS—Intragastric administration of indomethacin induced linear haemorrhagic mucosal lesions, primarily in the corpus of the stomach that were first observed at six hours. These lesions continued to develop over the next six hours with maximal lesion scores and mucosal permeabilities at 12 hours. When indomethacin was administered to mice deficient in CD18, intercellular adhesion molecule 1 (ICAM-1), or P-selectin, there were significant decreases in lesion scores compared with their C57BL/6 controls. In addition, mucosal permeabilities were found to be significantly lower in CD18 or ICAM-1 deficient mice observed at 12 hours. CONCLUSION—Certain leucocyte and endothelial cell adhesion molecules are important determinants for full expression of indomethacin induced gastropathy. It is proposed that this modification of the mouse model may be useful for the investigation of other pathophysiological mechanisms of NSAID induced gastropathy. Keywords: indomethacin; gastropathy; cyclooxygenase; intercellular adhesion molecule; VCAM; vascular cell adhesion molecule; P-selectin
Full Text
The Full Text of this article is available as a PDF (152.6 KB).
Figure 1 .
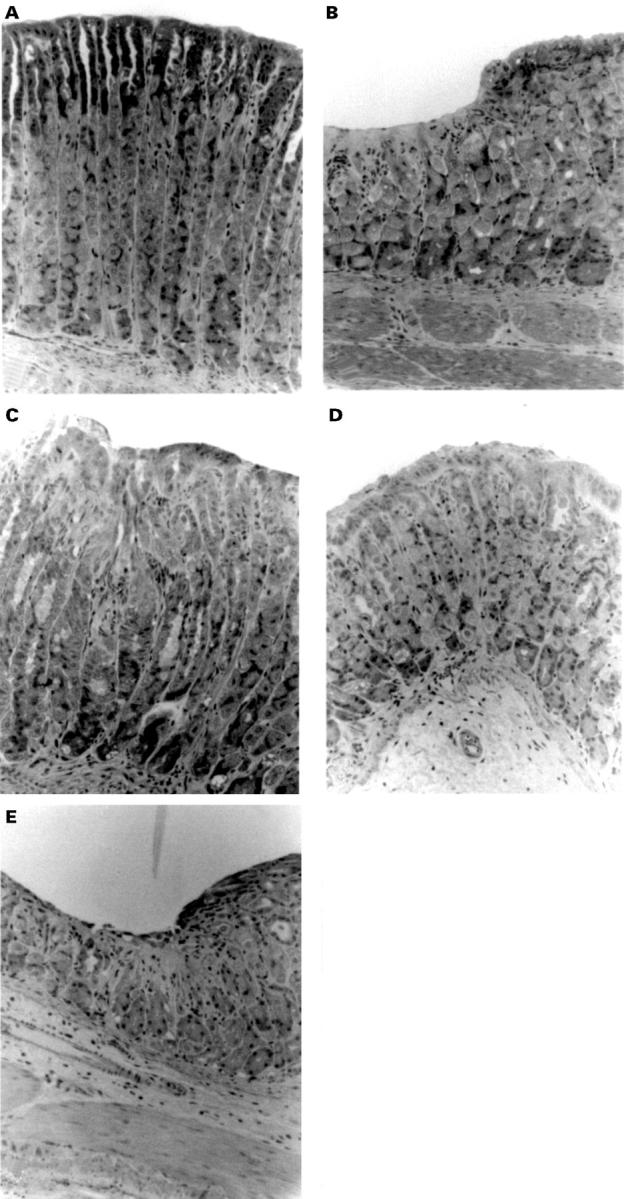
Histopathology of C57BL/6 mouse gastric mucosa resulting from indomethacin treatment. (A) Normal (untreated) mucosa. Typical distribution of apical located surface and mucous neck cells and more basal located chief and parietal cells. (B) Indomethacin treated mucosa of the wild type C57BL/6 mouse. Typical lesion showing erosion of the mucosa with predominant loss of the surface and mucous neck cells and loss of the integrity of the apical region of the glands. (C) Indomethacin treated mucosa of the CD18 deficient C57BL/6 mouse. Typical mucosa showing substantial protection to indomethacin treatment. The apical region of the glands appears disorganised, but does not exhibit erosion. (D) Indomethacin treated mucosa of the P-selectin deficient C57BL/6 mouse. Typical mucosa showing moderate protection to indomethacin treatment. The apical region of the glands appears disorganised with some general loss of surface mucous and mucous neck cells. Distinct regions of erosive lesion are not apparent. (E) Indomethacin treated mucosa of the ICAM-1 deficient C57BL/6 mouse. The absence of ICAM-1 expression had little protective effect. Typical lesions are found showing substantial erosion of the mucosa with loss of the integrity of the glands and loss of cells. However, ICAM-1 deficiency seemed to reduce the number of lesions.
Figure 2 .
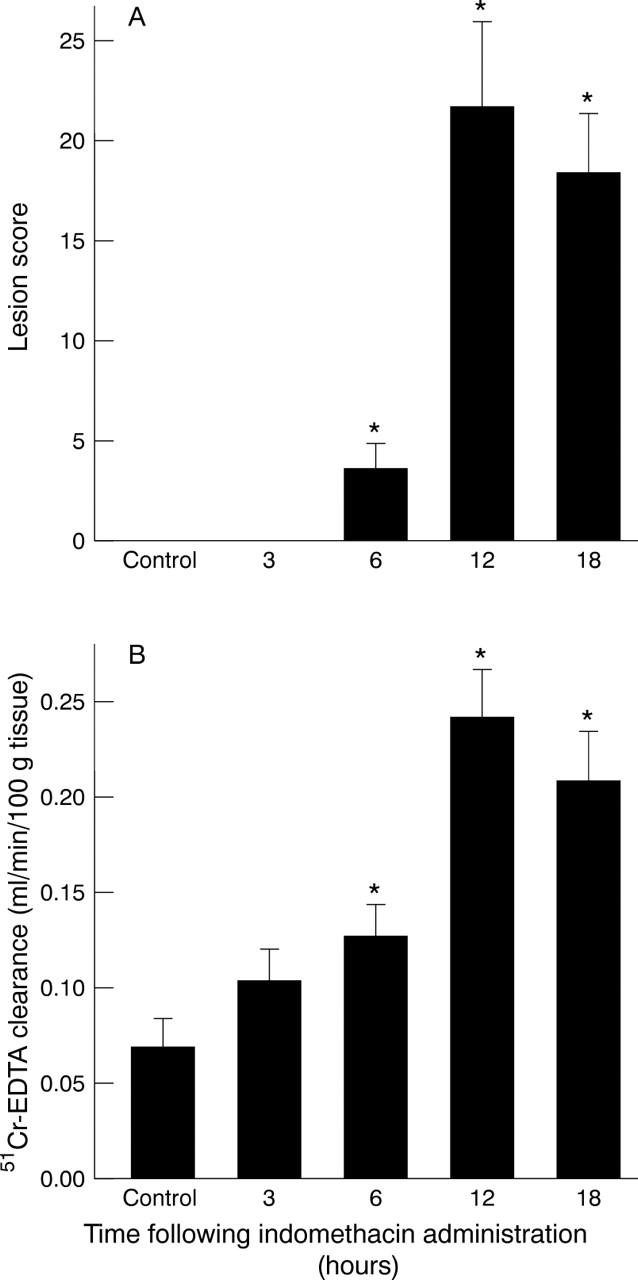
Time course of gastric mucosal lesion score (A) and mucosal permeability (B) following oral administration of indomethacin (20 mg/kg) to healthy wild type (C57BL/6) mice. *p<0.05 versus 0 hour.
Figure 3 .
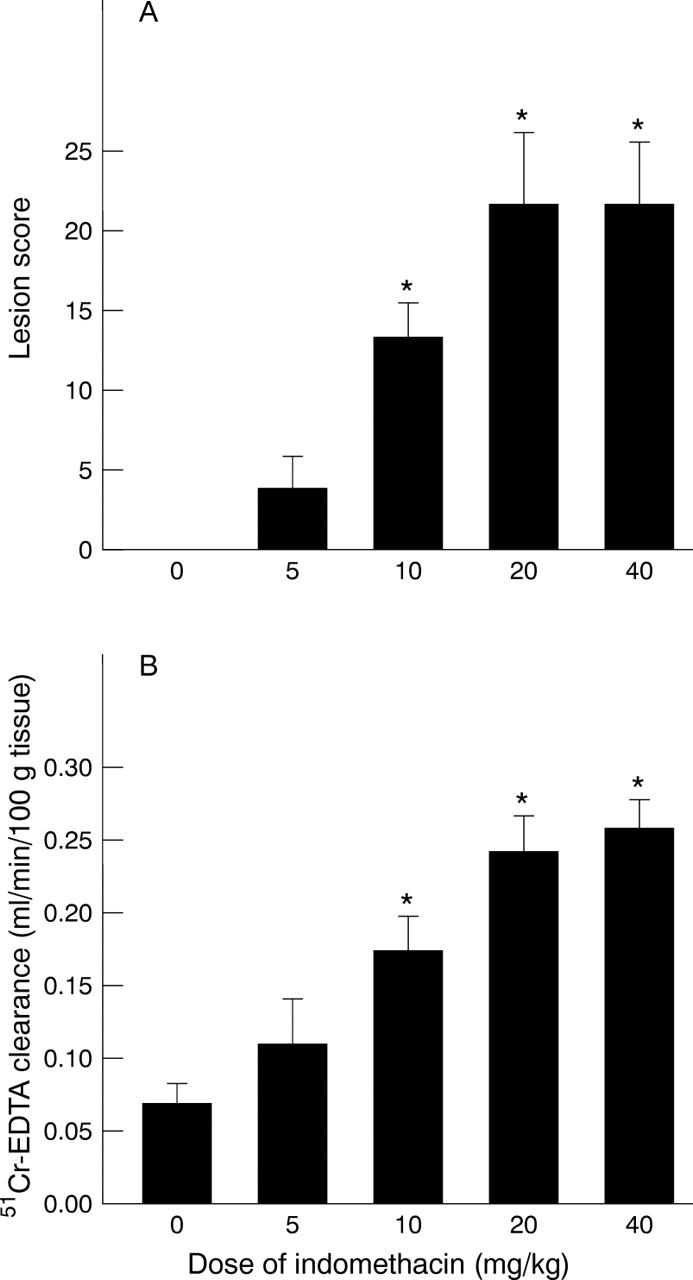
Gastric mucosal lesions (A) and mucosal permeability (B) induced by varying doses of indomethacin to healthy wild type mice. *p<0.05 versus control.
Figure 4 .
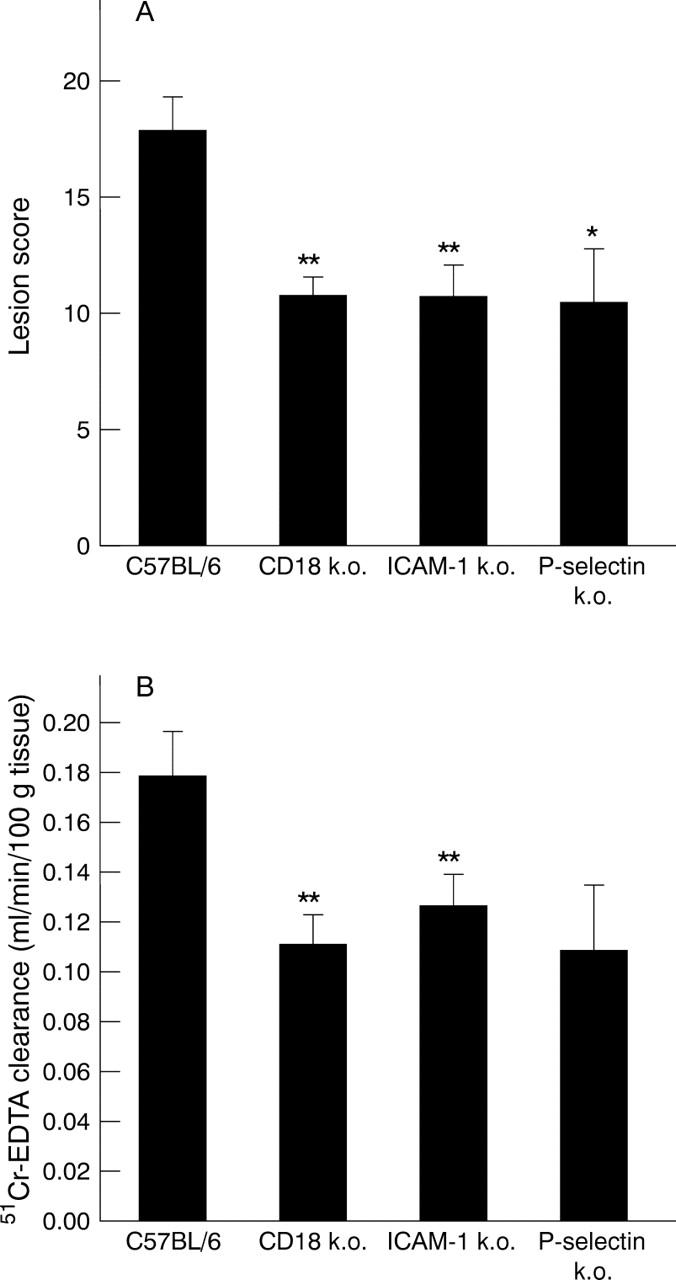
Gastric mucosal lesion score (A) and mucosal permeability (B) in wild type (C57BL/6), ICAM-1, CD18, and P-selectin deficient mice 12 hours following oral administration of indomethacin (20 mg/kg). *p<0.05 and **p<0.01 versus C57BL/6 mice.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eppihimer M. J., Russell J., Anderson D. C., Wolitzky B. A., Granger D. N. Endothelial cell adhesion molecule expression in gene-targeted mice. Am J Physiol. 1997 Oct;273(4 Pt 2):H1903–H1908. doi: 10.1152/ajpheart.1997.273.4.H1903. [DOI] [PubMed] [Google Scholar]
- Furie B., Furie B. C. Leukocyte crosstalk at the vascular wall. Thromb Haemost. 1997 Jul;78(1):306–309. [PubMed] [Google Scholar]
- Holland J., Owens T. Signaling through intercellular adhesion molecule 1 (ICAM-1) in a B cell lymphoma line. The activation of Lyn tyrosine kinase and the mitogen-activated protein kinase pathway. J Biol Chem. 1997 Apr 4;272(14):9108–9112. doi: 10.1074/jbc.272.14.9108. [DOI] [PubMed] [Google Scholar]
- Horie Y., Wolf R., Anderson D. C., Granger D. N. Hepatic leukostasis and hypoxic stress in adhesion molecule-deficient mice after gut ischemia/reperfusion. J Clin Invest. 1997 Feb 15;99(4):781–788. doi: 10.1172/JCI119224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahora T., Guth P. H. Effect of aspirin plus hydrochloric acid on the gastric mucosal microcirculation. Gastroenterology. 1987 Oct;93(4):810–817. doi: 10.1016/0016-5085(87)90444-6. [DOI] [PubMed] [Google Scholar]
- Komatsu S., Grisham M. B., Russell J. M., Granger D. N. Enhanced mucosal permeability and nitric oxide synthase activity in jejunum of mast cell deficient mice. Gut. 1997 Nov;41(5):636–641. doi: 10.1136/gut.41.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenbach R., Morham S. G., Tiano H. F., Loftin C. D., Ghanayem B. I., Chulada P. C., Mahler J. F., Lee C. A., Goulding E. H., Kluckman K. D. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995 Nov 3;83(3):483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- Ligumsky M., Sestieri M., Karmeli F., Zimmerman J., Okon E., Rachmilewitz D. Rectal administration of nonsteroidal antiinflammatory drugs. Effect on rat gastric ulcerogenicity and prostaglandin E2 synthesis. Gastroenterology. 1990 May;98(5 Pt 1):1245–1249. [PubMed] [Google Scholar]
- Mayadas T. N., Johnson R. C., Rayburn H., Hynes R. O., Wagner D. D. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993 Aug 13;74(3):541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- Morise Z., Komatsu S., Fuseler J. W., Granger D. N., Perry M., Issekutz A. C., Grisham M. B. ICAM-1 and P-selectin expression in a model of NSAID-induced gastropathy. Am J Physiol. 1998 Feb;274(2 Pt 1):G246–G252. doi: 10.1152/ajpgi.1998.274.2.G246. [DOI] [PubMed] [Google Scholar]
- Rainsford K. D. Gastric ulcerogenicity of non-steroidal anti-inflammatory drugs in mice with mucosa sensitized by cholinomimetic treatment. J Pharm Pharmacol. 1987 Aug;39(8):669–672. [PubMed] [Google Scholar]
- Rainsford K. D., Willis C. Relationship of gastric mucosal damage induced in pigs by antiinflammatory drugs to their effects on prostaglandin production. Dig Dis Sci. 1982 Jul;27(7):624–635. doi: 10.1007/BF01297219. [DOI] [PubMed] [Google Scholar]
- Rioux K. P., Wallace J. L. Mast cells do not contribute to nonsteroidal anti-inflammatory drug-induced gastric mucosal injury in rodents. Aliment Pharmacol Ther. 1996 Apr;10(2):173–180. doi: 10.1046/j.1365-2036.1996.724116000.x. [DOI] [PubMed] [Google Scholar]
- Rothlein R., Kishimoto T. K., Mainolfi E. Cross-linking of ICAM-1 induces co-signaling of an oxidative burst from mononuclear leukocytes. J Immunol. 1994 Mar 1;152(5):2488–2495. [PubMed] [Google Scholar]
- Sligh J. E., Jr, Ballantyne C. M., Rich S. S., Hawkins H. K., Smith C. W., Bradley A., Beaudet A. L. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8529–8533. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaananen P. M., Meddings J. B., Wallace J. L. Role of oxygen-derived free radicals in indomethacin-induced gastric injury. Am J Physiol. 1991 Sep;261(3 Pt 1):G470–G475. doi: 10.1152/ajpgi.1991.261.3.G470. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Arfors K. E., McKnight G. W. A monoclonal antibody against the CD18 leukocyte adhesion molecule prevents indomethacin-induced gastric damage in the rabbit. Gastroenterology. 1991 Apr;100(4):878–883. doi: 10.1016/0016-5085(91)90259-n. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Keenan C. M., Granger D. N. Gastric ulceration induced by nonsteroidal anti-inflammatory drugs is a neutrophil-dependent process. Am J Physiol. 1990 Sep;259(3 Pt 1):G462–G467. doi: 10.1152/ajpgi.1990.259.3.G462. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., McKnight W., Miyasaka M., Tamatani T., Paulson J., Anderson D. C., Granger D. N., Kubes P. Role of endothelial adhesion molecules in NSAID-induced gastric mucosal injury. Am J Physiol. 1993 Nov;265(5 Pt 1):G993–G998. doi: 10.1152/ajpgi.1993.265.5.G993. [DOI] [PubMed] [Google Scholar]
- Whittle B. J. Temporal relationship between cyclooxygenase inhibition, as measured by prostacyclin biosynthesis, and the gastrointestinal damage induced by indomethacin in the rat. Gastroenterology. 1981 Jan;80(1):94–98. [PubMed] [Google Scholar]
- Wilson R. W., Ballantyne C. M., Smith C. W., Montgomery C., Bradley A., O'Brien W. E., Beaudet A. L. Gene targeting yields a CD18-mutant mouse for study of inflammation. J Immunol. 1993 Aug 1;151(3):1571–1578. [PubMed] [Google Scholar]


