Abstract
BACKGROUND—The anti-idiotypic monoclonal antibody 105AD7 mimics the tumour associated antigen 791Tgp72, expressed on 70-80% of colorectal cancers. Phase I studies have shown that the vaccine is non-toxic, and a number of patients have been immunised prior to resection of their primary tumours. AIMS—To assess lymphocyte activation at the tumour site by measuring expression of the α subunit of the interleukin 2 receptor (CD25). METHODS—Nineteen patients with primary colorectal cancer were immunised with varying doses of 105AD7 prior to resection of their primary tumours. Samples of normal bowel and tumour edge/centre from 16 patients were available for immunohistochemical staining with a monoclonal antibody against CD25. Samples from a matched control group were also stained. Fresh tumours from 14 immunised patients and 31 unimmunised control patients were disaggregated, and the lymphocytes obtained labelled for CD25. Samples were analysed blindly by flow cytometry. RESULTS—Median infiltration of lymphocytes expressing CD25, measured immunohistochemically, was higher in trial patients, as was the ratio of tumour to normal bowel infiltration. Flow cytometric analysis of fresh tumour from immunised patients showed a significantly higher percentage of lymphocytes expressing CD25 tumour infiltrating lymphocytes than their matched and unmatched controls. DISCUSSION—The α subunit of the interleukin 2 receptor is increased on tumour infiltrating lymphocytes, in patients immunised with the colorectal cancer vaccine 105AD7. This suggests a population of activated lymphocytes capable of targeting 791Tgp72 expressing tumour cells, such as circulating micrometastases. 105AD7 may have a role as adjuvant therapy in early stage disease. Keywords: anti-idiotypic antibody; colorectal cancer; immunotherapy; 105AD7
Full Text
The Full Text of this article is available as a PDF (179.5 KB).
Figure 1 .
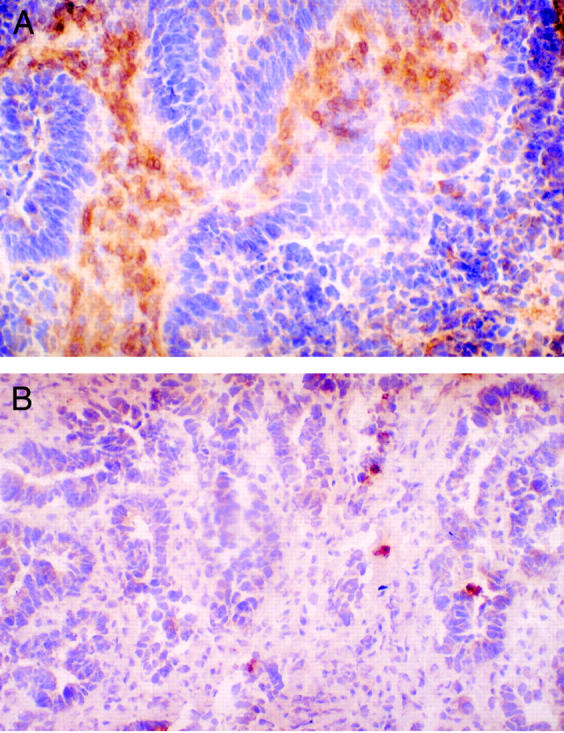
Infiltration of tumour centre by lymphocytes expressing CD25 in patients receiving 105AD7 (A), and their matched controls (B). Original magnification × 200.
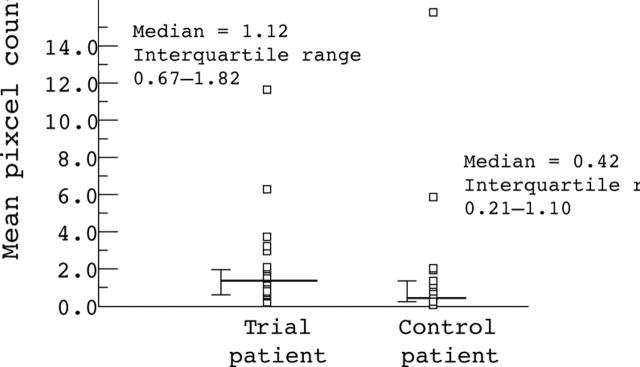
Figure 2 .
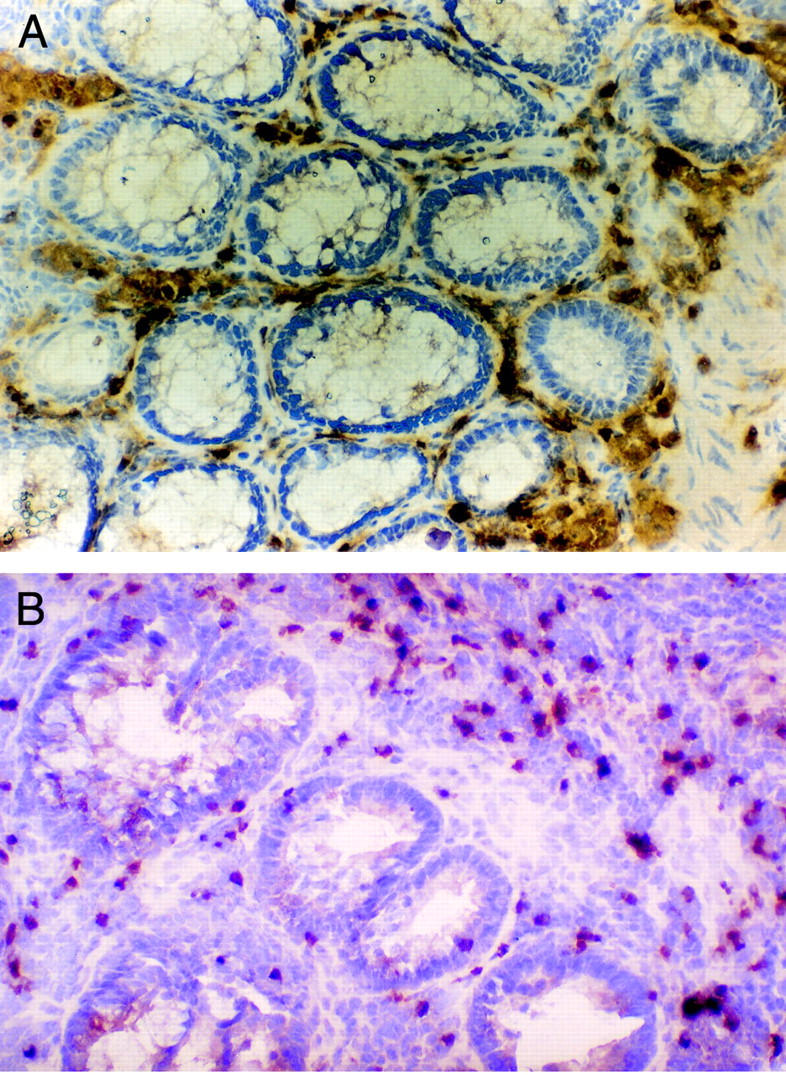
Infiltration by lymphocytes expressing CD25, at the tumour edge of trial patients who had received 105AD7 (A), and their matched controls (B). Original magnification × 200.
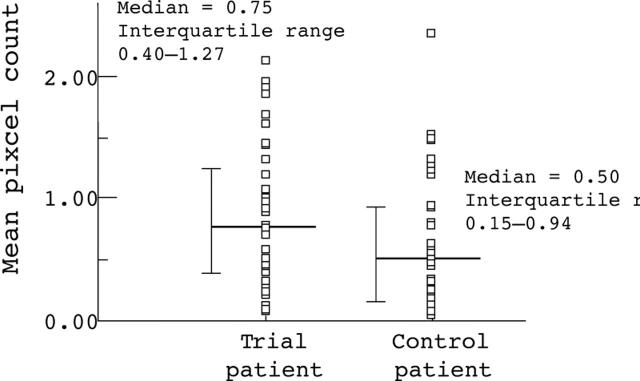
Figure 3 .
Immunohistochemical staining of tumour infiltrating lymphocytes expressing CD25 in trial and control patients, as measured by image analysis.
Figure 4 .
Tumour to normal bowel ratio of CD25 expressing lymphocytes.
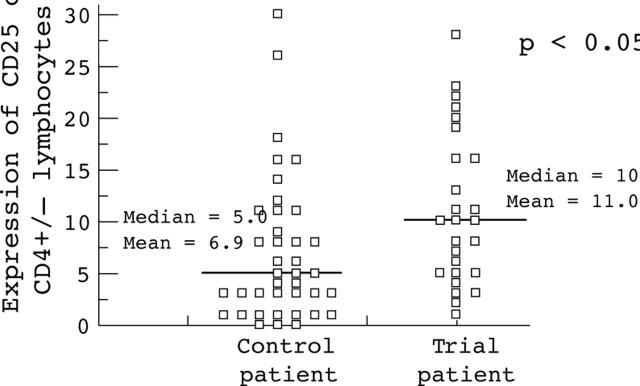
Figure 5 .
Percentage expression of CD25 on CD4+ and CD4− lymphocytes on tumour infiltrating lymphocytes, as measured by flow cytometry, in patients receiving 105AD7 preoperatively, and unimmunised controls.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen C., Hogg N. Elevation of infiltrating mononuclear phagocytes in human colorectal tumors. J Natl Cancer Inst. 1987 Mar;78(3):465–470. [PubMed] [Google Scholar]
- Austin E. B., Robins R. A., Baldwin R. W., Durrant L. G. Induction of delayed hypersensitivity to human tumor cells with a human monoclonal anti-idiotypic antibody. J Natl Cancer Inst. 1991 Sep 4;83(17):1245–1248. doi: 10.1093/jnci/83.17.1245. [DOI] [PubMed] [Google Scholar]
- Austin E. B., Robins R. A., Durrant L. G., Price M. R., Baldwin R. W. Human monoclonal anti-idiotypic antibody to the tumour-associated antibody 791T/36. Immunology. 1989 Aug;67(4):525–530. [PMC free article] [PubMed] [Google Scholar]
- Berd D., Maguire H. C., Jr, Mastrangelo M. J., Murphy G. Activation markers on T cells infiltrating melanoma metastases after therapy with dinitrophenyl-conjugated vaccine. Cancer Immunol Immunother. 1994 Sep;39(3):141–147. doi: 10.1007/BF01533378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley D. T., Robins A. R., Durrant L. G. Clinical evidence that the human monoclonal anti-idiotypic antibody 105AD7, delays tumor growth by stimulating anti-tumor T-cell responses. Hum Antibodies Hybridomas. 1995;6(2):68–72. [PubMed] [Google Scholar]
- Denton G. W., Durrant L. G., Hardcastle J. D., Austin E. B., Sewell H. F., Robins R. A. Clinical outcome of colorectal cancer patients treated with human monoclonal anti-idiotypic antibody. Int J Cancer. 1994 Apr 1;57(1):10–14. doi: 10.1002/ijc.2910570103. [DOI] [PubMed] [Google Scholar]
- Durrant L. G., Buckley T. J., Denton G. W., Hardcastle J. D., Sewell H. F., Robins R. A. Enhanced cell-mediated tumor killing in patients immunized with human monoclonal antiidiotypic antibody 105AD7. Cancer Res. 1994 Sep 15;54(18):4837–4840. [PubMed] [Google Scholar]
- Harel-Bellan A., Bertoglio J., Quillet A., Marchiol C., Wakasugi H., Mishall Z., Fradelizi D. Interleukin 2 (IL 2) up-regulates its own receptor on a subset of human unprimed peripheral blood lymphocytes and triggers their proliferation. J Immunol. 1986 Apr 1;136(7):2463–2469. [PubMed] [Google Scholar]
- Håkansson L., Adell G., Boeryd B., Sjögren F., Sjödahl R. Infiltration of mononuclear inflammatory cells into primary colorectal carcinomas: an immunohistological analysis. Br J Cancer. 1997;75(3):374–380. doi: 10.1038/bjc.1997.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob L., Somasundaram R., Smith W., Monos D., Basak S., Marincola F., Pereira S., Herlyn D. Cytotoxic T-cell clone against rectal carcinoma induced by stimulation of a patient's peripheral blood mononuclear cells with autologous cultured tumor cells. Int J Cancer. 1997 May 2;71(3):325–332. doi: 10.1002/(sici)1097-0215(19970502)71:3<325::aid-ijc3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Maxwell-Armstrong C. A., Durrant L. G., Scholefield J. H. Colorectal cancer vaccines. Br J Surg. 1998 Feb;85(2):149–154. doi: 10.1046/j.1365-2168.1998.00704.x. [DOI] [PubMed] [Google Scholar]
- McMillan D. N., Kernohan N. M., Flett M. E., Heys S. D., Deehan D. J., Sewell H. F., Walker F., Eremin O. Interleukin 2 receptor expression and interleukin 2 localisation in human solid tumor cells in situ and in vitro: evidence for a direct role in the regulation of tumour cell proliferation. Int J Cancer. 1995 Mar 16;60(6):766–772. doi: 10.1002/ijc.2910600606. [DOI] [PubMed] [Google Scholar]
- Miescher S., Whiteside T. L., Carrel S., von Fliedner V. Functional properties of tumor-infiltrating and blood lymphocytes in patients with solid tumors: effects of tumor cells and their supernatants on proliferative responses of lymphocytes. J Immunol. 1986 Mar 1;136(5):1899–1907. [PubMed] [Google Scholar]
- Rees A. D., Scoging A., Dobson N., Praputpittaya K., Young D., Ivanyi J., Lamb J. R. T cell activation by anti-idiotypic antibody: mechanism of interaction with antigen-reactive T cells. Eur J Immunol. 1987 Feb;17(2):197–201. doi: 10.1002/eji.1830170208. [DOI] [PubMed] [Google Scholar]
- Robins R. A., Denton G. W., Hardcastle J. D., Austin E. B., Baldwin R. W., Durrant L. G. Antitumor immune response and interleukin 2 production induced in colorectal cancer patients by immunization with human monoclonal anti-idiotypic antibody. Cancer Res. 1991 Oct 1;51(19):5425–5429. [PubMed] [Google Scholar]
- Smith K. A. The interleukin 2 receptor. Annu Rev Cell Biol. 1989;5:397–425. doi: 10.1146/annurev.cb.05.110189.002145. [DOI] [PubMed] [Google Scholar]
- Trinchieri G., Matsumoto-Kobayashi M., Clark S. C., Seehra J., London L., Perussia B. Response of resting human peripheral blood natural killer cells to interleukin 2. J Exp Med. 1984 Oct 1;160(4):1147–1169. doi: 10.1084/jem.160.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose B. M., Vánky F., Klein E. Human tumour--lymphocyte interaction in vitro. V. Comparison of the reactivity of tumour-infiltrating, blood and lymph-node lymphocytes with autologous tumour cells. Int J Cancer. 1977 Dec 15;20(6):895–902. doi: 10.1002/ijc.2910200612. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Goldman C. K., Robb R. J., Depper J. M., Leonard W. J., Sharrow S. O., Bongiovanni K. F., Korsmeyer S. J., Greene W. C. Expression of interleukin 2 receptors on activated human B cells. J Exp Med. 1984 Nov 1;160(5):1450–1466. doi: 10.1084/jem.160.5.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. M., Smith K. A. The interleukin 2 receptor. Functional consequences of its bimolecular structure. J Exp Med. 1987 Oct 1;166(4):1055–1069. doi: 10.1084/jem.166.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann E., Sacchi M., Plaisance S., Heo D. S., Yasumura S., Lin W. C., Johnson J. T., Herberman R. B., Azzarone B., Whiteside T. L. Receptors for interleukin 2 on human squamous cell carcinoma cell lines and tumor in situ. Cancer Res. 1992 Nov 1;52(21):5963–5970. [PubMed] [Google Scholar]